Creative Biolabs can offer reverse yeast two-hybrid (rY2H) system services for detection of protein-protein interactions. As a genetic method, rY2H has been widely used in drug screening.
Protein-protein interactions take part in most biological processes of cells including gene expression, cell growth, proliferation, morphology, motility, intercellular communication. And the use of yeast two-hybrid (Y2H) system has provided definition to previously unknown pathways by the characterization of novel interactions between proteins. The two-hybrid system relies on the bifunctional nature of transcription factors, such as yeast enhancer Gal4 or repressor LexA to allow protein-protein interactions to be monitored through transcriptional changes of reporter genes. When a positive interaction has been validated, either of the interacting proteins can be mutated by site-specific or randomly introduced changes, to generate proteins with a decreased capability to interact. Such technique is termed reverse two-hybrid (rY2H/rYTH) method, specifically designed to promote identification of events isolating protein-protein interactions. More attention will shift to this powerful search engine for the establishment of interaction networks as well as drug discovery in understanding of diseases.
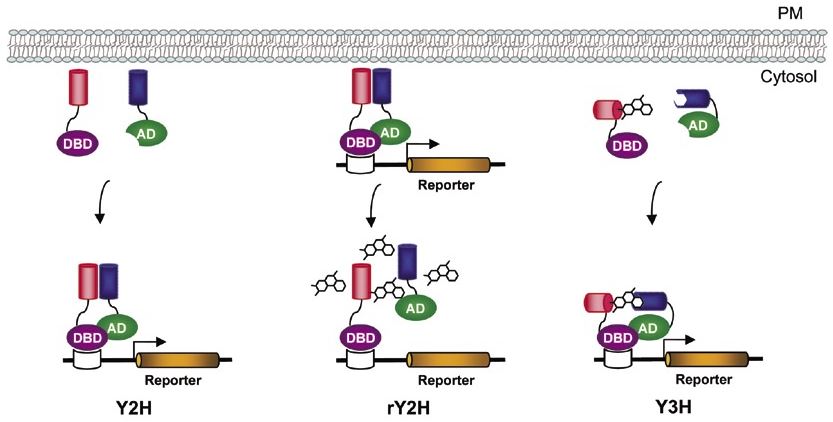 Fig.1 Reverse yeast two-hybrid (rY2H) for drug screening.
Fig.1 Reverse yeast two-hybrid (rY2H) for drug screening.
The reverse two-hybrid system is a genetic scheme that has far been described to discern molecules or mutations that dissociate specific interacting partners for a protein of interest. In Saccharomyces cerevisiae, yeast strains are engineered so that the interaction of two proteins expressed in the context of the counterselective system is deleterious to growth. Under these conditions, dissociating interactome provides an alternative survival advantage, thereby facilitating detection. This method contributes to the study of the structure-function relationships and regulation of protein-protein interactions. It also should accelerate the selection of dissociator which can be used as therapeutic agents.
Conceptually, rY2H makes use of a contrary strategy to produce a positive readout for disruption of the designated interactions of proteins. Hence, no parallel toxicity testing of the complexes is required in the screening procedure. That is to say, in this upside-down version of Y2H, the wild-type protein interaction is toxic or lethal for the yeast cells because of a toxic marker (e.g. URA3). In this setting, dissociation confers a selective growth advantage that can easily identify both interacting proteins and trans-acting dissociators or small molecules. The lynchpin of rY2H is the incorporation of a reporter gene, to monitor protein-protein interactions, whose final products are poisonous to the living cells. This allows the utilization of selective pressure against the formation of two-hybrid compounds.
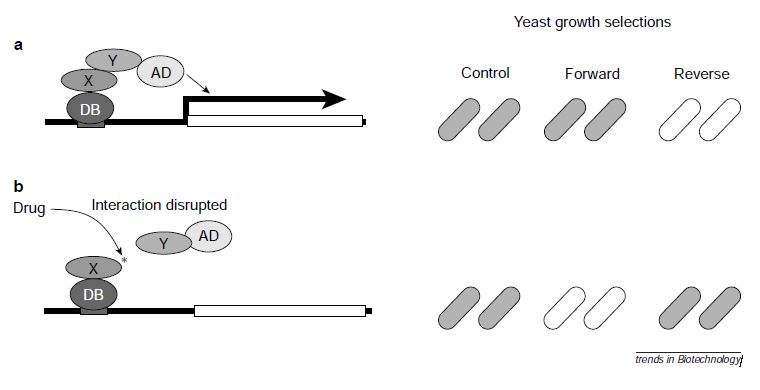 Fig.2 An illustration of the way two-hybrid systems work.
Fig.2 An illustration of the way two-hybrid systems work.
The yeast gene URA3 encodes an enzyme involved in uracil biosynthesis and transforms 5-flouroorotic acid (5-FOA) into a deleterious metabolite that leads to cell death. Therefore, yeast cells that express URA3 fail to grow on media containing 5-FOA and gene URA3 is commonly chosen as a counter-selectable marker (negative selection). There is a yeast strain engineered in which expression of URA3 is controlled by a strictly regulated promoter containing GAL4 binding sites. Expression of interacting GAL4 activation domain (AD) with GAL4 DNA-binding domain (DBD) rescues the viability of this strain on media lacking uracil, while growth on complete media adding 5-FOA is inhibited by interacting AD and DBD fusions. As a result, dissociating mutations in interacting proteins can be isolated from a large library of randomly generated mutants by selection for 5-FOA-resistant colonies.
Proteins are basic building components of cells and protein interactions and define key biochemical and regulatory networks. Protein-protein interactions are an essential aspect of all signaling pathways and biological mechanisms and are strongly predictive of functional relationships.
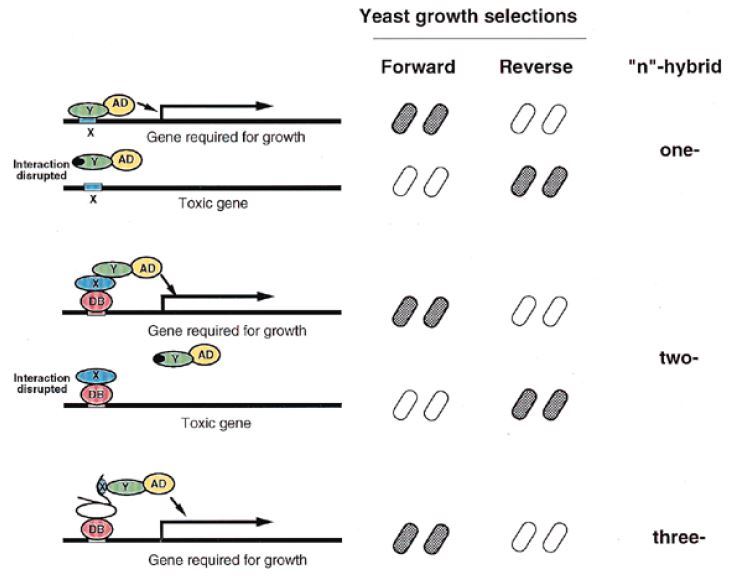 Fig.3 Forward and reverse n-hybrid system.
Fig.3 Forward and reverse n-hybrid system.
Theoretically, in all diseases attributed to particular protein-protein interactions, special dissociation can be regarded as a potential therapeutic strategy. Identification of dissociator molecules could be achieved by direct selection through the reverse two-hybrid system. Creative Biolabs has experienced and advanced platforms in rY2H methods that are applied for abrogating defined macromolecular interactions and rational drug-screening. Besides that, Creative Biolabs can still offer other forward or reverse “n”-hybrid systems professionally to evaluate the complex interplay of proteins with DNAs, RNAs, peptide ligands, small organic ligands, protein kinases, etc.
Other optional two-hybrid systems:
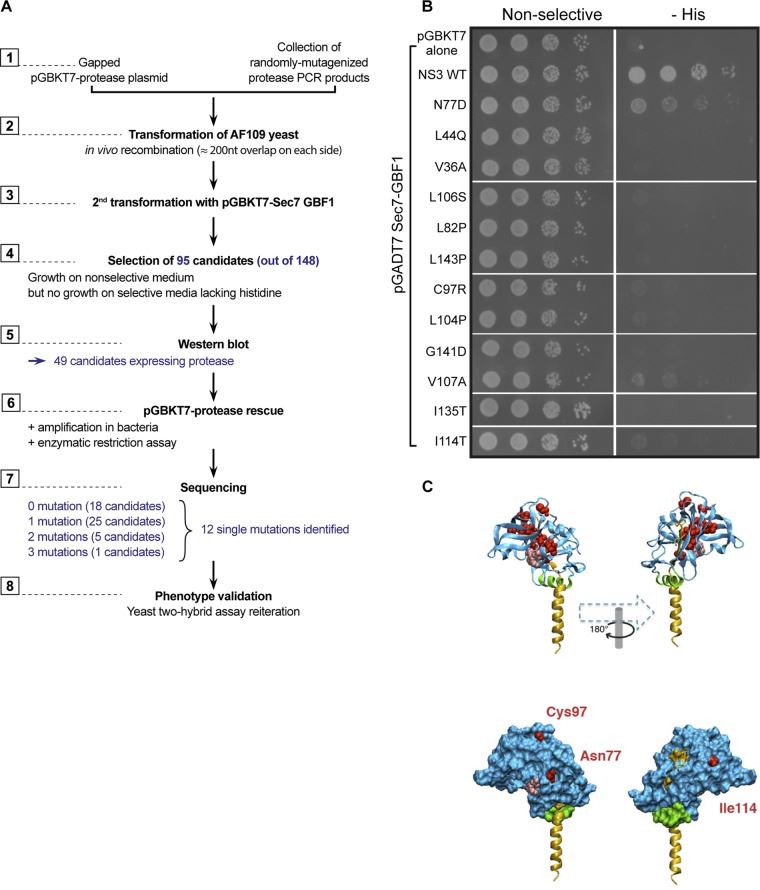 Fig. 4 Interaction of Sec7‐GBF1 with HCV NS3 protease mutants. (Nadjet Lebsir, 2019)
Fig. 4 Interaction of Sec7‐GBF1 with HCV NS3 protease mutants. (Nadjet Lebsir, 2019)
GBF1 has become the host factor for the genomic replication of different families of RNA viruses. In the life cycle of the hepatitis C virus (HCV), GBF1 plays a key role at the beginning of genome replication. To understand how GBF1 regulates HCV infection, the researchers studied the interaction between GBF1 and HCV proteins. Through yeast two-hybrid, co-immunoprecipitation, and adjacent ligation assays, it was found that NS3 interacted with GBF1, interfered with the function of GBF1, and changed the intracellular location of GBF1 in the cells expressing NS3. In addition, NS3 mutants altering the NS3-GBF1 interaction were identified by a reverse yeast two-hybrid screening assay. Although its protease activity was retained, it did not interact with GBF1. The mutant residue was exposed on the surface of NS3, indicating that it is part of the NS3 domain that interacts with GBF1. The results show that the change in the interaction between NS3 and GBF1 is harmful to HCV genome replication.
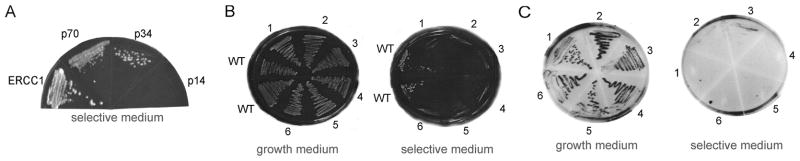 Fig. 5 Isolation of a mutant XPF that is defective in interaction with RPA. (Laura A. Fisher, 2011)
Fig. 5 Isolation of a mutant XPF that is defective in interaction with RPA. (Laura A. Fisher, 2011)
Nucleotide excision repair (NER) can resist various types of DNA damage and is essential for maintaining genome stability. Studies have shown that appropriate protein-protein interactions between NER factors are essential for effective repair. Structure-specific endonuclease XPF-ERCC1 can make a 5 incision in NER and has been shown to interact with RPA, a single-stranded DNA-binding protein. However, the biological significance of this interaction is not clear. The researchers used the yeast two-hybrid assay to determine the interaction between XPF and the p70 subunit of RPA. Next, a p70 interaction deficient XPF was isolated by the reverse yeast two-hybrid assay using random mutagenesis XPF. The mutant XPF contained an amino acid substitution at the N terminal. Compared with wild-type XPF, the expression of this mutant XPF in the XPF-deficient Chinese hamster ovary cell line only partially restored NER activity and UV resistance in vivo. At the same time, it was found that RPA interaction defective XPF was not located in the nucleus, while the mislocalization of XPF-ERCC1 prevented the complex from playing a role in NER.
The reverse yeast two-hybrid (rY2H) system is an adaptation of the conventional yeast two-hybrid (Y2H) system designed to identify inhibitors of protein-protein interactions (PPIs). While the traditional Y2H system is used to detect the interaction between two proteins, the rY2H system is utilized to screen for molecules that can disrupt these interactions. This makes it particularly useful in drug discovery and functional genomics.
In the rY2H system, two proteins that are known to interact are genetically fused to two different reporter genes. These fusion constructs are then introduced into yeast cells. If the proteins interact, the reporter genes are activated, leading to a detectable change, typically a growth or color change in the yeast. To identify disruptors of the interaction, libraries of small molecules, peptides, or mutant versions of the interacting proteins are introduced into this system. Compounds or mutations that prevent the interaction cause the reporter gene not to activate, thus inhibiting the detectable change and indicating potential inhibitors of the PPI.
The reverse yeast two-hybrid system is extensively used in research and drug development for its ability to identify inhibitors of protein-protein interactions, which are often key players in many diseases, including cancer, viral infections, and neurodegenerative disorders. By finding molecules that can disrupt these interactions, the rY2H system provides a pathway for the development of new therapeutic agents.
One of the primary applications of rY2H is in the identification of novel drug targets. By disrupting specific protein-protein interactions and observing the effects on the cell or organism, researchers can identify potential targets for drug action. This system is also employed in studying the function of various proteins within cellular pathways, helping to elucidate the mechanisms of diseases at a molecular level. Furthermore, the rY2H system can be used in synthetic biology for constructing novel protein networks, allowing researchers to explore new ways proteins can be engineered to perform specific functions within cells.
High Throughput Capability: The rY2H system allows for the screening of large libraries of compounds or genetic variants, making it an efficient method to identify interactions or inhibitors of interactions on a large scale. This high throughput nature is crucial for drug discovery, where many potential inhibitors need to be screened quickly.
Cost-Effectiveness: Yeast as a model organism is inexpensive to maintain and manipulate genetically. This cost efficiency makes the rY2H system accessible for various laboratories, including those with limited resources.
Versatility: The system can be used with a wide range of proteins, including those from humans and other organisms. This versatility makes it suitable for studying a vast array of biological processes and diseases.
Sensitivity and Specificity: The rY2H system is highly sensitive to detecting protein-protein interactions and their disruption. It can identify even weak interactions, which might be overlooked by other methods.
Construct Design: The first step is to construct the DNA sequences encoding the proteins of interest, fused to the DNA-binding and activation domains of the transcription factors. These constructs must be designed carefully to ensure they do not affect the native interaction of the proteins.
Yeast Transformation: The constructs are then introduced into yeast cells using transformation techniques such as electroporation or chemical transformation. Efficient transformation is critical to ensuring a sufficient number of yeast cells carry the constructs for reliable results.
Library Screening: To identify disruptors of the protein interaction, a library of potential inhibitors (such as small molecules, peptides, or mutant proteins) is also introduced into the yeast cells. This library can be a collection of known chemicals, a genetically engineered library of protein variants, or a random peptide library.
Selection and Screening: Yeast cells are grown under conditions where only cells in which the interaction is disrupted will survive or exhibit a change (such as loss of color or fluorescence). Surviving colonies are then isolated for further analysis.
Validation: Hits from the primary screen are validated through retesting in yeast and, ideally, followed up with additional tests in more complex systems, like mammalian cells, to confirm the biological relevance of the interaction disruption.
The reverse yeast two-hybrid system can be effectively integrated with other biotechnological and analytical methods to enhance its utility and overcome some of its limitations:
Mass Spectrometry: Following the identification of potential PPI disruptors, mass spectrometry can be used to analyze the interaction and identify the binding sites and mechanisms at a molecular level.
High-content Screening: Integration with high-content screening allows for the simultaneous analysis of multiple cellular parameters, providing a richer set of data about the biological effects of disrupting a particular protein interaction.
CRISPR/Cas9: CRISPR technology can be used to introduce the genes encoding the fusion proteins directly into the genome of the yeast, leading to more stable expression and potentially more relevant physiological interactions.
Computational Modeling: Computational tools can predict which interactions are most likely to be biologically significant and thus worthy of further investigation in the rY2H system.
By combining the rY2H system with these technologies, researchers can gain a more comprehensive understanding of protein interactions and their inhibitors, facilitating the development of targeted therapies and enhancing our understanding of cellular processes.
The reverse yeast two-hybrid (rY2H) system is versatile and can be used to screen a wide variety of molecular types for their ability to disrupt protein-protein interactions.
Small Molecules: Small molecules are often the primary candidates screened in rY2H assays due to their potential as therapeutic drugs. Their diverse structures and ability to penetrate cells make them ideal for modulating biological pathways.
Peptides: Peptides are another class of molecules that can be screened using the rY2H system. They can mimic or block protein interaction interfaces and are useful in validating the specific sites of protein interactions.
Antibodies: Antibodies that can specifically bind to one of the proteins in the interaction pair can potentially disrupt the interaction. Screening for such antibodies can be valuable in therapeutic applications, particularly in diseases where specific protein interactions are known to play a role.
RNA Molecules: RNA-based molecules, including siRNA and miRNA, can be used to alter protein expression levels, indirectly affecting protein-protein interactions. Screening for RNAs that result in the disruption of target interactions can provide insights into gene regulation and protein function.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
