Protein-protein interactions (PPIs) stand at the heart of most cellular processes and control the vast majority of biological systems, especially in mediating functions such as sensing the environment, mediating signal transduction, and adjusting the activity of metabolic and signaling enzymes. Protein-protein interaction (PPI) has already become one of the major objectives in structural biology and life science. Nowadays, Creative Biolabs contributes various experimental and bioinformatic studies to helping scientists deepen understanding of protein interactions and improve our capacity to predict them.
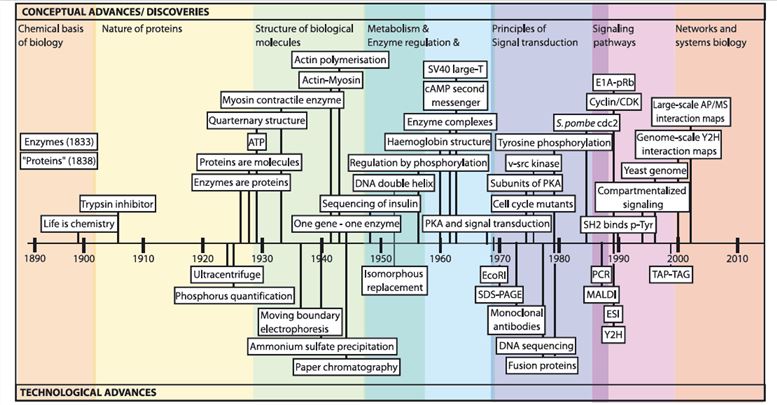 Fig.1 Timeline of protein-protein interaction research. (Braun, 2012)
Fig.1 Timeline of protein-protein interaction research. (Braun, 2012)
Protein-protein interactions are physical contacts of specificity established between two or more protein molecules and caused by electrostatic forces including the hydrophobic effect. Based on their characteristics, PPIs can be classified in several ways. Considering their interaction surface, they may be homo- or heterooligomeric; as measured by stability, they could be obligate or nonobligate; when judged by their persistence, they may be transient or permanent. A given protein’s interaction may be a combination of these three specific pairs.
In brief, PPIs fundamentally could be characterized as stable or transient. Both stable and transient interactions can be either strong or weak, fast or slow. The transient interactions would form signaling pathways while stable ones will produce a stable protein complex. Generally, stable interactions are best detected by co-immunoprecipitation, pull-down or far-Western assays and transient interactions can be captured by crosslinking or label transfer methods.
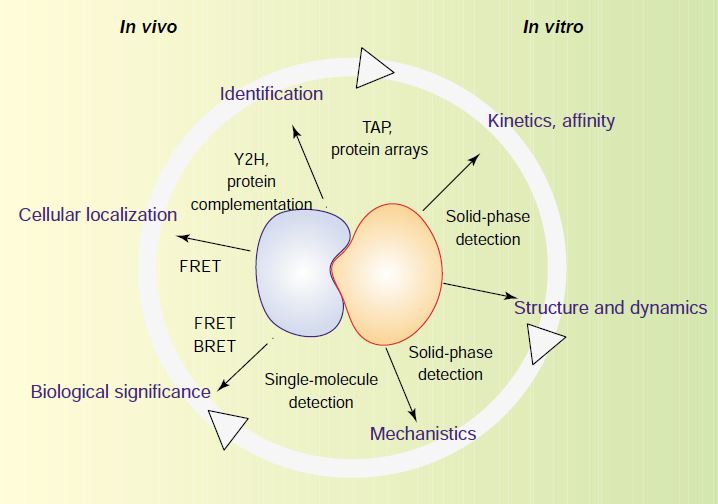 Fig.2 Different levels of characterization of protein interactions in vivo and in vitro. (Piehler, 2005)
Fig.2 Different levels of characterization of protein interactions in vivo and in vitro. (Piehler, 2005)
In Creative Biolabs, PPI detection approaches are classified into three types, in vivo, in vitro, and in silico methods.
| Experimental Methods | ||
| In vivo | Yeast two-hybrid (Y2H) | Bacterial two-hybrid (B2H) system |
| Y2H-based Drug-Target Interaction Identification | Fluorescence resonance energy transfer (FRET) | |
| Bioluminescence resonance energy transfer (BRET) | Proximity-dependent Biotin Identification (BioID) | |
| Bimolecular fluorescence complementation (BiFC) | TurboID | |
| Synthetic lethality | Yeast Three-Hybrid (Y3H) for Protein Interaction Identification | |
| In vitro | Tandem affinity purification (TAP) | Affinity chromatography |
| Co-immunoprecipitation (Co-IP) | Pull-down assay | |
| Protein arrays | Protein-fragment complementation assay (PCA) | |
| Phage display | X-ray crystallography | |
| Surface plasmon resonance (SPR) | Nuclear magnetic resonance spectroscopy (NMR spectroscopy) | |
| In silico | Gene fusion | Sequence-based approaches |
| Chromosome proximity | Structure-based approaches | |
| In silico two hybrid | Mirror tree | |
| Phylogenetic tree | Gene expression-based approaches | |
Table 1. Protein-protein interaction services.
Besides the conventional and widely used approaches to detect protein-protein interaction, we have also developed several novel platforms to meet specific requirements, such as Magic™ Conversable Mammalian Library (CML) Platform for Biomolecular Interaction Discovery.
The result of two or more proteins that interact with each other can be demonstrated in different ways. The measurable biological effects of PPIs have been outlined as follows:
PPIs handle a wide range of biological processes, ranging from cell-to-cell interactions to metabolic and developmental control. At present, PPI is one of the key topics for the development and progress of modern systems biology. Creative Biolabs focuses on all available biochemical methods and advanced biophysical techniques for identifying associated protein molecules and dissecting cellular protein interactors functions under well-defined conditions. Furthermore, usually a combination of techniques is necessary to test, validate and confirm protein interactions in an experimental design of Creative Biolabs. In this respect, data and information obtained from many different perspectives are much more reliable and comprehensive.
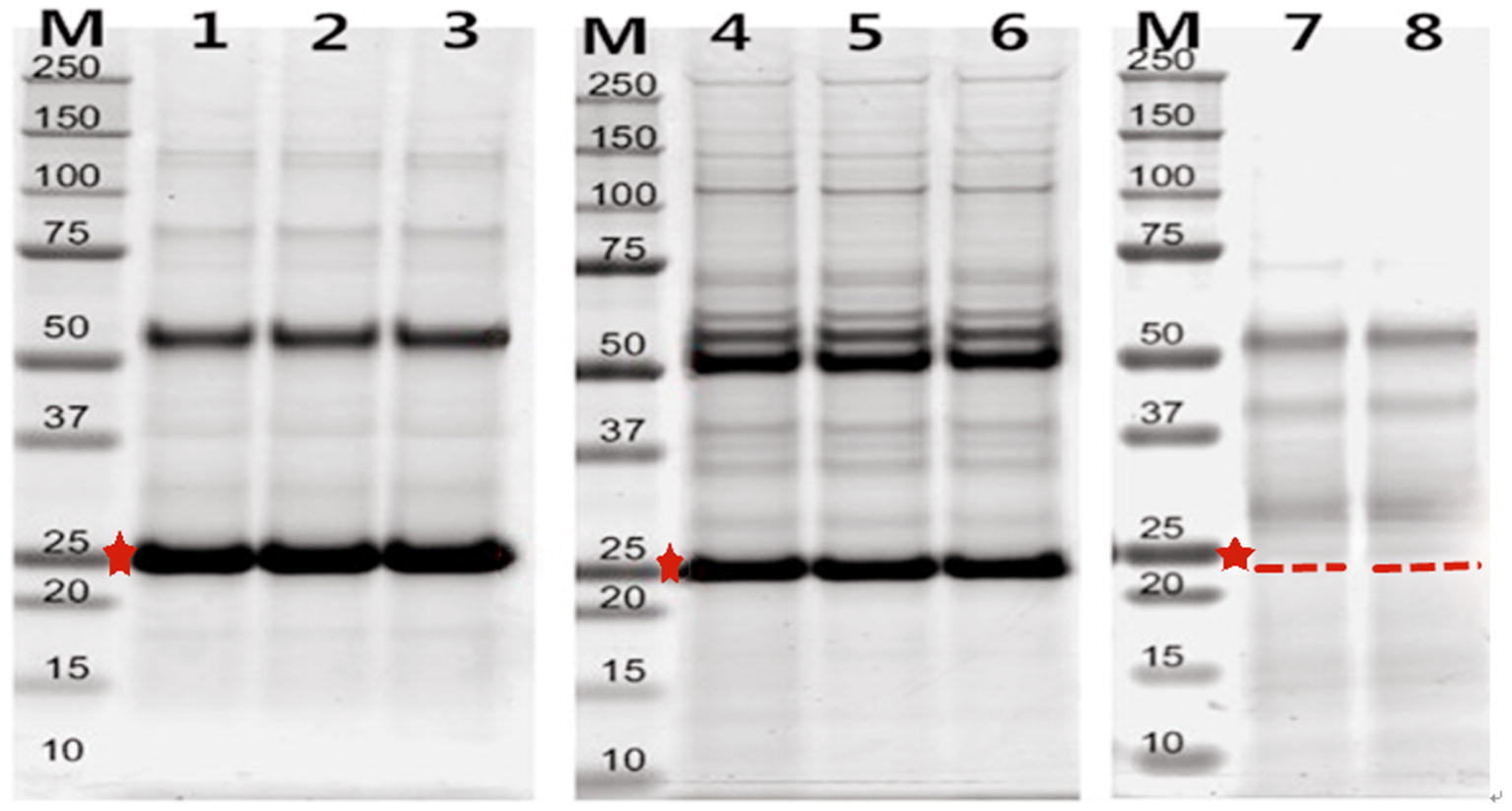 Fig. 3 Identification of BMPR-1B/FecB interacting proteins. (Jianlei Jia, 2020)
Fig. 3 Identification of BMPR-1B/FecB interacting proteins. (Jianlei Jia, 2020)
BMPR-1B is a part of the transforming growth factor β superfamily and plays a key role in the litter size of ewes. In this paper, the researchers constructed the eukaryotic expression system, prepared monoclonal antibodies, and characterized BMPR-1B/FecB protein-protein interactions (PPIs). Using co-immunoprecipitation coupled to mass spectrometry (Co-IP/MS), they identified 23 proteins that specifically interact with FecB in ewe ovarian extracts. Bioinformatics analysis of selected PPI showed that FecB and its related interacting proteins enriched the reproductive process through BMP2 and BMP4 pathways. Through the analysis of biological processes and pathways, it was determined that the signal transduction is carried out through the Smads protein and TGF-β signal pathway. In addition, other target proteins (GDF5, GDF9, RhoD, and HSP 10) interacting with FecB and related to ovulation and litter size in ewes were identified.
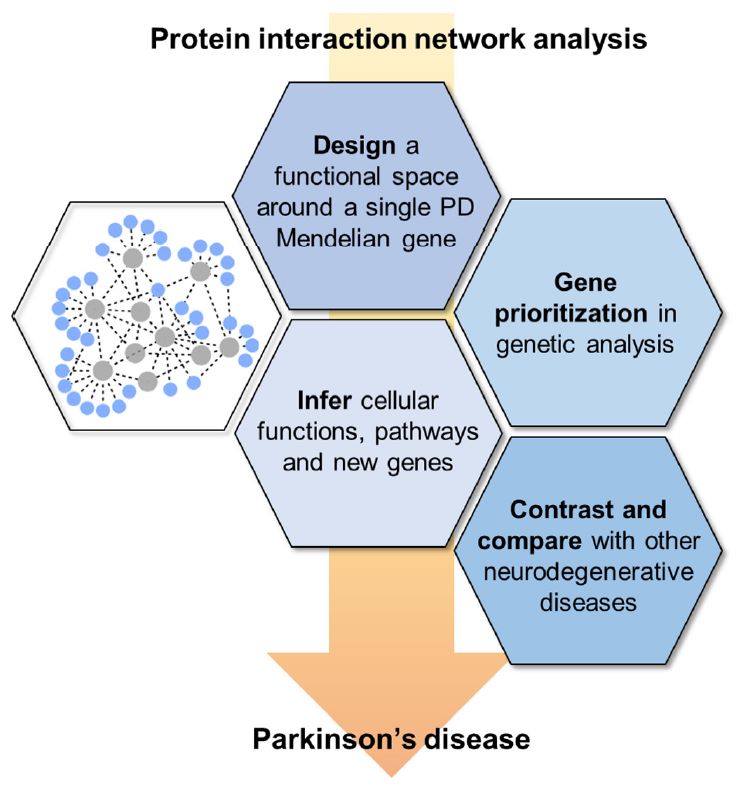 Fig. 4 Different PPI network approaches, defined based on the target goals of the study in which they are employed. (James E Tomkins, 2021)
Fig. 4 Different PPI network approaches, defined based on the target goals of the study in which they are employed. (James E Tomkins, 2021)
Protein-protein interaction is a key component of subcellular molecular networks that enable cells to function. Because of their importance in balance in the body, changes in the network can be harmful, leading to cell dysfunction and eventually a disease state. Parkinson's disease is a progressive neurodegenerative disease, and its etiology involves many factors. PPIs network analysis can accelerate the understanding of molecular crosstalk and biological processes behind the pathogenesis of PD. In this review, the researchers describe the practicability of PPI network methods in complex system modeling, focusing on previous PD research work. They discussed four main strategies for using the PPI network approach: a, infer PD-related cellular functions, pathways, and new genes; b, support genomics research; c, study the interaction groups of individual PD-related genes; and d, compare PD with the molecular basis of other neurodegenerative diseases. This is a growing area of research that may be further expanded with the increase in the generation and availability of combinatorial data. These methods complement and bridge the gap between genetic and functional research and provide information for future research.
PPI assays are pivotal in the field of drug discovery, particularly for identifying and validating new therapeutic targets. By elucidating the interaction networks between proteins, researchers can pinpoint crucial interactions that regulate biological processes and disease states. Inhibitors or modulators of specific protein-protein interactions can be developed as potential therapeutic agents. For instance, disrupting interactions involved in signal transduction pathways can inhibit the progression of diseases like cancer. Moreover, PPI assays help in the screening of large libraries of compounds to find molecules that can specifically disrupt these interactions.
Protein-protein interactions can be detected and analyzed using various methods, each with its advantages and limitations.
Yeast Two-Hybrid (Y2H) System: This genetic method uses yeast cells to detect PPIs by reconstituting a functional transcription factor when two proteins interact, leading to a detectable reporter gene expression.
Co-Immunoprecipitation (Co-IP): This biochemical method involves the use of antibodies to precipitate one protein and, subsequently, any proteins bound to it, which can then be detected by Western blotting.
Fluorescence Resonance Energy Transfer (FRET): A biophysical technique that measures the energy transfer between two fluorophore-labeled molecules (proteins) when in close proximity, indicating interaction.
Surface Plasmon Resonance (SPR): This method measures the binding of proteins to each other by detecting changes in the refractive index near the surface of a sensor chip on which one of the proteins is immobilized.
The specificity and affinity of protein-protein interactions can be quantitatively assessed using various analytical techniques, such as:
Isothermal Titration Calorimetry (ITC): This technique directly measures the heat released or absorbed during the binding of two proteins, providing data on the stoichiometry, affinity, and thermodynamics of the interaction.
Surface Plasmon Resonance (SPR): By flowing one protein over a sensor chip with another protein immobilized, SPR can provide real-time binding kinetics, from which the association and dissociation rates (ka and kd) and the equilibrium dissociation constant (Kd) can be calculated.
Microscale Thermophoresis (MST): This technique monitors the movement of proteins in a temperature gradient, which changes upon binding to another protein, allowing for the determination of binding affinities.
Bioluminescence Resonance Energy Transfer (BRET): Similar to FRET, BRET measures energy transfer between proteins tagged with a bioluminescent donor and a fluorescent acceptor, which can be used to calculate interaction dynamics.
The outcome of PPI assays can be significantly influenced by various environmental conditions such as pH, temperature, and the presence of cofactors or other interacting molecules.
pH and Ionic Strength: Changes in pH can alter the charge and folding of proteins, potentially affecting their ability to interact. Similarly, ionic strength can influence electrostatic interactions between proteins.
Temperature: Protein conformation and dynamics are temperature-dependent, which can impact interaction affinity and specificity. Each protein may have an optimal temperature range for stable interactions.
Cofactors and Post-translational Modifications: The presence of cofactors required for protein activity or modifications like phosphorylation can be essential for some interactions to occur. Therefore, mimicking physiological conditions during PPI assays is crucial for accurate results.
Complexity of Protein Structures: Many proteins undergo conformational changes upon binding or may only be stable in complex cellular environments. This makes it difficult to study these interactions in vitro or in simplified systems like the yeast two-hybrid system.
False Positives and False Negatives: PPI assays can sometimes yield false positive results due to non-specific interactions, or false negatives if the assay conditions do not faithfully replicate the native cellular environment or miss transient interactions.
Dynamic Range of Detection: The sensitivity of detecting weak or transient interactions can be limited by the dynamic range of the assay method. This is particularly challenging in methods like co-immunoprecipitation, where weakly interacting or rapidly dissociating complexes may not be effectively captured.
How are advancements in technology improving PPI assays?
High-Throughput Screening (HTS): Automation and miniaturization have enabled the screening of thousands of interactions in a single experiment, using techniques such as array-based systems and automated fluorescence microscopy.
Proximity Labeling: Techniques like BioID and APEX use enzyme-mediated labeling of proximal proteins in living cells, allowing for the identification of interaction partners in their native cellular context.
Computational Predictions and Machine Learning: Computational tools and machine learning are increasingly being used to predict PPIs based on protein structure data, genetic information, and known interaction networks, which can guide experimental verification.
Protein-protein interactions are fundamental to the operation of cellular signaling pathways. PPI assays enable researchers to map out the networks of interactions that underpin signal transduction within the cell. By identifying the specific partners that proteins interact with, these assays help elucidate the mechanisms by which signals are relayed, amplified, or terminated inside cells. This understanding is crucial for pinpointing where signaling pathways might be disrupted in diseases such as cancer or neurodegenerative disorders, providing potential targets for therapeutic intervention.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
