Based on the technology of B cell sorting, Creative Biolabs provides Native™ human antibody discovery service to generate native human antibodies for therapeutic use. These antibodies are derived from B cells of individuals possessing natural immunity to certain diseases. They have affinity-matured, in vivo human immune responses and with no sequence modifications to alter binding affinity. We believe that native human antibodies will become the new generation of therapeutic mAbs.
Monoclonal antibodies (mAbs) are essential tools for many diagnostic applications and research investigations. In addition, mAbs have become ideal therapeutic reagents for many diseases and are well established in the treatment of cancer, autoimmune disorders, and infectious diseases. Many technologies exist for the generation of mAbs including hybridoma, phage display. Generally, there have been four generations of therapeutic mAbs generated.
Four Generations of mAbs
1st generation-Murine antibodies: the first generation of therapeutic antibodies were developed from mice about twenty years ago. For now, there are still several murine mAbs available for treatment of diseases. However, as foreign substances, murine mAbs trigger immunoreaction and the generation of anti-drug antibodies in vivo. The immunogenicity of murine mAbs limits their effectiveness as therapeutics.
2nd generation-Chimeric antibodies: chimeric antibodies possess the variable domains (VH and VL) of murine mAbs and human antibody constant regions. The chimeric antibodies have lower immunogenicity and have achieved commercial success.
3rd generation-Humanized antibodies: even after chimeric antibodies became widespread, the immunogenicity was still an issue, which resulted in the development of the humanized antibodies from 1986. Humanization is the process to increase the “human” content in an antibody. The main techniques include CDR grafting, SDR grafting, and superhumanization.
4th generation-Fully human antibodies: several sophisticated approaches including humanization help with further reduction of murine content in therapeutic antibodies. More recently, “fully human antibodies” are catching the attention of pharmacy industry. The so-called “fully human antibodies” are generated by phage display of antibody library and then affinity maturation in vitro.
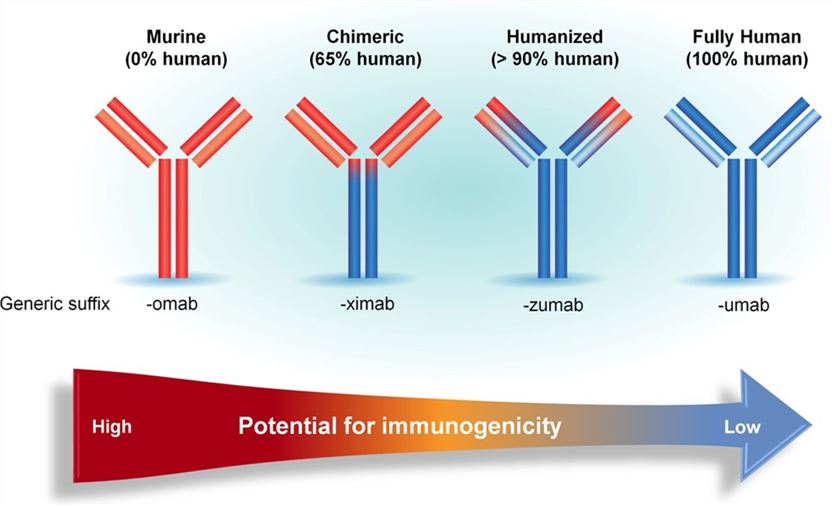 Fig.1 Four Generations of mAbs and their corresponding immunogenicity.1
Fig.1 Four Generations of mAbs and their corresponding immunogenicity.1
However, these high-affinity antibodies are not germline encoded but rather are derived through recombination and somatic hypermutation of genomic DNA. Since mature antibody sequences are not encoded in the germline, they can be regarded as foreign proteins. So, actually, there are no true human antibodies marketed at present.
The next generation of mAbs would be native human antibodies which are derived directly from a human gene and ensure faithful reproduction of the original human gene. The usage of B cell sorting makes it easier and effective to generate such antibodies.
The Advantages of Native Human Antibodies
Cooperating with our partners, scientists from Creative Biolabs assist our clients to develop natural human antibodies derived from individuals possessing natural immunity to certain diseases.
Through this novel platform, Creative Biolabs can isolate naïve-paired monoclonal antibodies from a wide range of host species:
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
