Diagnosing Exosomes of COVID-19
Exosomes can carry cargoes of proteins, lipids and non-coding RNAs involved in mediating COVID-19-induced infection, pro-inflammation and tissue damage in the lung and respiratory tract, thus necessitating experiments and studies as diagnostic markers of disease. Creative Biolabs can provide not only the most professional customization services for exosome products, but also exosome research services related to COVID-19, helping clients from all over the world to advance their COVID-19 research.
Mechanism of Viral Infection of COVID-19
The global pandemic of novel coronavirus disease 2019 (COVID-19) has spawned a global health crisis that has been urgently addressed in recent years. As of today, the development of therapeutic agents targeting the suppression of coronavirus-2 (SARS-CoV-2) infection is receiving attention from researchers worldwide, one of which is the emerging exosome-based diagnostic and therapeutic strategies.
SARS-CoV-2 is an enveloped virus composed of spike (S), envelope (E), membrane (M), nucleocapsid (N) proteins and other accessory proteins, and contains a 29.9 kb positive-stranded RNA genome. SARS-CoV-2 can cause disease and species cross-transmission in birds and mammals, and in humans it mainly infects the lungs, causing tissue damage and dysfunction in the lungs and other organs after an incubation period of 5 to 14 days. As a donor organ for blood oxygenation and decarboxylation necessary to support aerobic life, the lungs maintain normal respiratory function with a strictly regulated immune and inflammatory response, while lung epithelial cells, as virus-susceptible cells, have a highly expressed angiotensin-converting enzyme 2 (ACE2) on their surface that acts as an acceptor and interacts with the viral envelope protein, and under the action of transmembrane protease serine 2 (TMPRSS2). The S protein fuses with the cell membrane under the action of transmembrane protease serine 2 (TMPRSS2), thus completing the internalization of SARS-CoV-2 and the production of viral polypeptide. Finally, SARS-CoV-2 becomes host-dependent for maturation and self-replication, leaves the host and infects new cells at scale.
Diagnosing Exosomes of COVID-19
Clinical observations have shown that patients with COVID-19 have an inflammatory response and dysregulated immune response in the lungs and in many extra-pulmonary organ systems, including but not limited to the heart, nervous system, and urinary system due to a pro-inflammatory cytokine storm caused by viral inhibition of interferon signaling, particularly interleukin-6 (IL-6) and tumor necrosis factor-α (TGF-α). Given the interaction with SARS-CoV-2, exosomes have the potential to be used as one of the diagnostic markers of COVID-19. Exosomes, as natural lipid bilayer enclosed structural vesicles, are produced by infected cells in the respiratory tract and share structural and physicochemical similarities with viruses secreted by infected cells, facilitating virus infection, maturation and multiplication. By means of the process of exosome formation, secretion and extracellular dissemination, SARS-CoV-2 is able to accomplish dissemination and cellular infection between infected and uninfected cells, and can facilitate the secretion and composition of vesicles such as exosomes. Thus, exosomes mediate intercellular communication and dissemination, and are capable of carrying soluble mediators produced by virus induction in infected cells to distant organs and tissues.
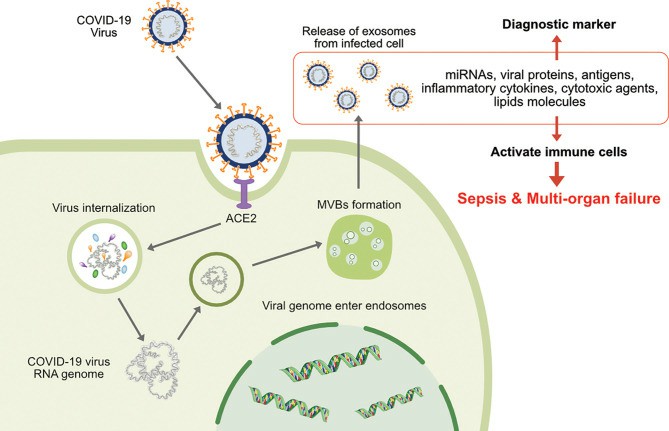 Fig.1 The involvement of exosomes on transmission, infection and release of virion particles and contents of diagnostic makers of COVID-19. (Gurunathan, 2021)
Fig.1 The involvement of exosomes on transmission, infection and release of virion particles and contents of diagnostic makers of COVID-19. (Gurunathan, 2021)
Exosomes resembling the mother cell state can be isolated from biofluid sources (e.g. plasma) and studied as biomarkers of COVID-19. There has been growing evidence to support the role of exosome-mediated COVID-19 in infection, transmission and reinfection in humans. An unbiased proteomic analysis has compared the plasma exosome composition of healthy volunteers and COVID-19 patients. The results show that plasma levels of Tenascin-C (TNC) and fibrinogen-β (FGB) are significantly higher in COVID-19 patients than in normal controls, and that pro-inflammatory cytokines can be stimulated by TNC and FGB production via the nuclear factor-κB (NF-κB) pathway, exhibiting cytokine storm and immune overload in COVID-19 patients. Moreover, a significant increase in IL-6, TNF-α and CCL5 levels was observed in hepatocytes exposed to exosomes from COVID-19 patients. Thus, plasma exosomes from COVID-19 patients can deliver substances such as TNC, TGB, and miRNA to cells in uninfected organs, trigger pro-inflammatory cytokine signals and expand areas of lesion damage, potentially serving as a novel marker for the diagnosis of diseases such as COVID-19.
 Fig.2 Schematic diagram represents interplay between infected cells and recipient cells through extracellular vesicles by various processes such as phagocytosis, macropinocytosis, endocytosis and fusion of viral particles. (Gurunathan, 2021)
Fig.2 Schematic diagram represents interplay between infected cells and recipient cells through extracellular vesicles by various processes such as phagocytosis, macropinocytosis, endocytosis and fusion of viral particles. (Gurunathan, 2021)
As one of the subtypes of extracellular vesicles, COVID-19 infected hosts secrete exosomes that exhibit molecular characteristics different from those secreted by uninfected cells, modulate host defense and immune evasion, and are important mediators of viral reactivation and reinfection. Studies have shown that exosomes carrying viral particles are one of the emerging diagnostic markers for pulmonary and respiratory diseases such as COVID-19. Whether it is the extraction, purification and identification of exosomes or the evaluation of functional effects such as diagnostics and therapeutics, Creative Biolabs is confident to provide you with customized exosome services related to COVID-19 studies. Please contact us to find out more information about your interest.
Reference
-
Gurunathan, S.; et al. Diverse effects of exosomes on COVID-19: a perspective of progress from transmission to therapeutic developments. Front Immunol. 2021, 12: 716407.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 The involvement of exosomes on transmission, infection and release of virion particles and contents of diagnostic makers of COVID-19. (Gurunathan, 2021)
Fig.1 The involvement of exosomes on transmission, infection and release of virion particles and contents of diagnostic makers of COVID-19. (Gurunathan, 2021)
 Fig.2 Schematic diagram represents interplay between infected cells and recipient cells through extracellular vesicles by various processes such as phagocytosis, macropinocytosis, endocytosis and fusion of viral particles. (Gurunathan, 2021)
Fig.2 Schematic diagram represents interplay between infected cells and recipient cells through extracellular vesicles by various processes such as phagocytosis, macropinocytosis, endocytosis and fusion of viral particles. (Gurunathan, 2021)








