Exosomes to Prevent COVID-19
The outbreak and global prevalence of coronavirus disease (COVID-19) in 2019 underscores the urgency of its disease prevention and vaccine development, while the safety of current adenoviral vector and lipid nanovector-based vaccines remains questionable, exosomes are very promising as ideal vectors for drug delivery and engineering customizable COVID-19 vaccines derived from them. With years of experience in exosome extraction, characterization and functional validation, Creative Biolabs is confident to continue to provide our customers with the most professional research services for exosome-based prevention of COVID-19.
Infection and Deterioration of COVID-19
The increasing global prevalence of COVID-19 and the high mutation rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has led to the emergence of new viral variants around the world, is one of the major challenges in controlling the COVID-19 situation. The genome has 79.6% and 50% sequence identity with SARS-CoV and Middle East Respiratory Syndrome (MERS)-CoV, respectively, and 88% high sequence identity with bat-SL-CoVZC45 and bat-SL-CoVZXC21, and can be transmitted by contaminated surface contact, fecal-oral transmission, and respiratory route, which is more efficient than SARS-CoV and The pathogenesis of COVID-19 begins in respiratory epithelial cells, and the 26s protein of SARS-CoV-2 can bind to angiotensin-converting enzyme 2 (ACE2) on the cell surface via the receptor binding domain (RBD), and its viral pathogenicity is increased after RBD mutation. Meanwhile, integrins can serve as alternative receptors for enveloped viruses to infect the host, and the tripeptide Arg-Gly-Asp (RGD) of SARS-CoV can complete replication and migrate to the airway dependent on the host after binding to integrins, and the release of viral particles into the intercellular space further promotes its dissemination and pathological changes, causing the onset of the organism's immune response. Finally, targeting targets are established in the systemic circulation through the hyaline membrane of the fibrin component of the virus, expanding the infection to include distant organs such as the intestine, kidney and bladder, causing lung injury and dysfunction of various organs in patients.
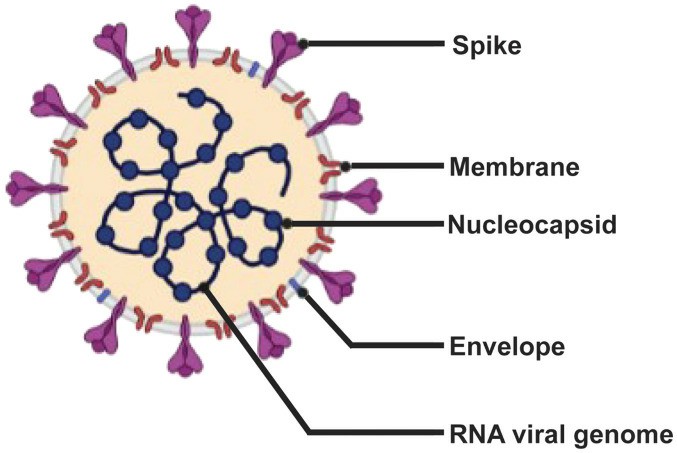 Fig.1 Schematic diagram of SARS-CoV-2. (Yoo, 2022)
Fig.1 Schematic diagram of SARS-CoV-2. (Yoo, 2022)
Exosome-based Prevention and Vaccine Development against COVID-19
With continued high incidence and transmission rates, the development and vaccination of COVID-19 vaccine has become an effective disease control measure to protect patients from future outbreaks of viral infection and limit mass transmission of the virus. Based on the research on the mechanism of SARS-CoV-2 infection, the development of COVID-19 vaccine mainly targets classical molecular targets such as the S protein or its RBD. Currently, although attenuated or inactivated viral vaccines based on viral vectors, mRNA, S protein and its subunits have been used for a long time, their protection duration and immunity effects are still limited, and the safety of adenoviral vectors is questionable, such as the occurrence of side effects like thrombocytopenia and coagulation. At the same time, the high mutation rate of the virus is continuously urging the need for alternative strategies in vaccine development, and the search for more effective antigen expression, longer duration of immune protection and safer vaccines is still necessary. Nowadays, scientists and researchers worldwide are turning their attention to the field of COVID-19 vaccine development based on extracellular vesicles (Evs) such as exosomes. For example, vaccine development strategies based on mRNA and full-length S proteins of chimerically modified S, N, M and E proteins in Evs. Phase I trials of engineered vaccines for EVs against the tumor cytokine IL-12 have demonstrated favorable safety and tolerability profiles. In addition, several EVs vaccine trials have reported that such engineered exosomal vaccines based on viral peptides not only stimulate the release of interferon-γ (IFN-γ) from CD8+ T cells in an antigen-specific manner, but also induce specific antibody titers higher than those in patient serum, restoring normal humoral and cell-mediated immune function and triggering long-lasting antiviral effects.
 Fig.2. Approaches to exosome-based vaccine in the management of COVID-19. (Yoo, 2022)
Fig.2. Approaches to exosome-based vaccine in the management of COVID-19. (Yoo, 2022)
More importantly, the ability of EVs to retain naive antigenic conformation, high vascular permeability, and high biosafety meet the criteria for an effective vaccine compared to other vectors such as viruses and lipid nanoparticles. For example, both in vitro and mouse studies showed that neither human embryonic kidney cell 293T-derived exosomes nor human mesenchymal stem cell-derived exosomes exhibited genotoxic and hematological effects. Genome-wide transcriptome analysis was performed on recipient Human hepatoma cells 24 hours after exposure to human embryonic kidney cells-derived CD81+/CD63+/CD9+ exosomes. The results showed no differential expression of genes associated with inflammation and toxicity, and no hepatotoxic or damaging histopathological changes in BALB/c mice after exosome injection. In summary, several of these results affirm that exosome-derived vaccines eliminate the adverse risk of viral vector vaccine-induced inflammation and could be ideal candidates for COVID-19 vaccines.
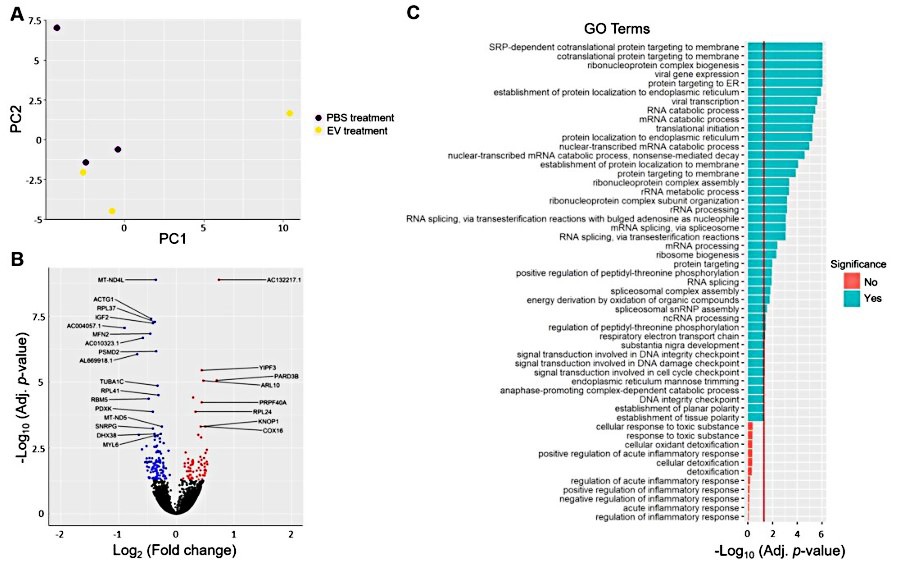 Fig.3 Transcriptional changes in HepG2 cells after EVs treatment. (Saleh, 2019)
Fig.3 Transcriptional changes in HepG2 cells after EVs treatment. (Saleh, 2019)
COVID-19 vaccines based on exosomes and other vesicle-derived vaccines can trigger the normal cellular and humoral immune effects of the host by loading SARS-CoV-2 structural chimeric proteins (S, E, M and N proteins) and their mRNAs, and have attracted the interest and attention of researchers working on COVID-19 worldwide. At the same time, the findings of high vascular permeability and low immunogenicity of exosomes support the prospect of exosomes in vaccine development. To help our clients with COVID-19 and exosome-related research, Creative Biolabs offers a range of services including exosome preparation, functional validation, and vaccine development related services. Please contact us for any of your related needs.
References
-
Yoo, K.H.; et al. Possibility of exosome‑based coronavirus disease 2019 vaccine (Review). Mol Med Rep. 2022, 25(1): 26.
-
Saleh, A.F.; et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale. 2019, 11(14): 6990-7001.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Schematic diagram of SARS-CoV-2. (Yoo, 2022)
Fig.1 Schematic diagram of SARS-CoV-2. (Yoo, 2022)
 Fig.2. Approaches to exosome-based vaccine in the management of COVID-19. (Yoo, 2022)
Fig.2. Approaches to exosome-based vaccine in the management of COVID-19. (Yoo, 2022)
 Fig.3 Transcriptional changes in HepG2 cells after EVs treatment. (Saleh, 2019)
Fig.3 Transcriptional changes in HepG2 cells after EVs treatment. (Saleh, 2019)








