Therapeutic Exosomes for COVID-19
Exosomes have similar carrier molecules and therapeutic properties to their source and are promising interventions for the study and development of drugs targeting COVID-19. Creative Biolabs has a long history of providing products and services related to exosome applications to customers from around the world conducting COVID-19 research.
Overview of COVID-19
COVID-19 is the third highly pathogenic coronavirus after SARS-CoV and Middle East Respiratory Syndrome Coronavirus (MERS-CoV). The novel coronavirus (NCoV) belongs to the order NidoVirales of the coronaviridae family and contains positive-stranded single-stranded RNA viruses ranging from 26 to 32 kb in length, with structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. The virus binds to the angiotensin-converting enzyme 2 (ACE-2) receptor, which is abundantly expressed on the surface of epithelial cells, and further self-replicates and infects cells with the help of the host, affecting the normal immune response regulation and causing extensive alveolar damage, resulting in pneumonia and progressive respiratory failure. Tomographic images of the patient's lungs show marked intra-alveolar edema, fibrin and collagen deposition, leukocyte infiltration and bronchiectasis, as well as elevated biochemical parameters such as neutrophils, total bilirubin, lactate deaminase and alanine aminotransferase, and severely reduced lymphocyte counts and albumin, accompanied by overactive T lymphocytes and reduced numbers of regulatory T cells.
Therapeutic Exosomes for COVID-19
The cytopathic effects and viral immune escape response triggered by COVID-19 determine the severity of the disease, and reports show that serum interferon-γ, IL-1β, 2, 4, 6, 8, 10, 17, inducible protein 10, monocyte chemotactic protein-1, granulocyte colony-stimulating factor, and tumor necrosis factor α levels are significantly higher in patients with severe neocoronary pneumonia than in those without severe disease, so T lymphocytes and macrophages at abnormal activation levels leading to immune dysregulation and progressive failure of respiratory system function. Numerous studies have shown that mesenchymal stem cells (MSCs) are known for their immunomodulatory properties, inducing the production of regulatory T cell (Treg) subsets and anti-inflammatory macrophages. In addition, the expression of low levels of major histocompatibility complex (MHC) class I molecules and the absence of MHC class II molecules make MSCs low immunogenic and ideal candidates for cell therapy, and the safety of MSCs of allogeneic origin, such as bone marrow, adipose tissue, Wharton's jelly and placenta, has been evaluated in clinical trials.
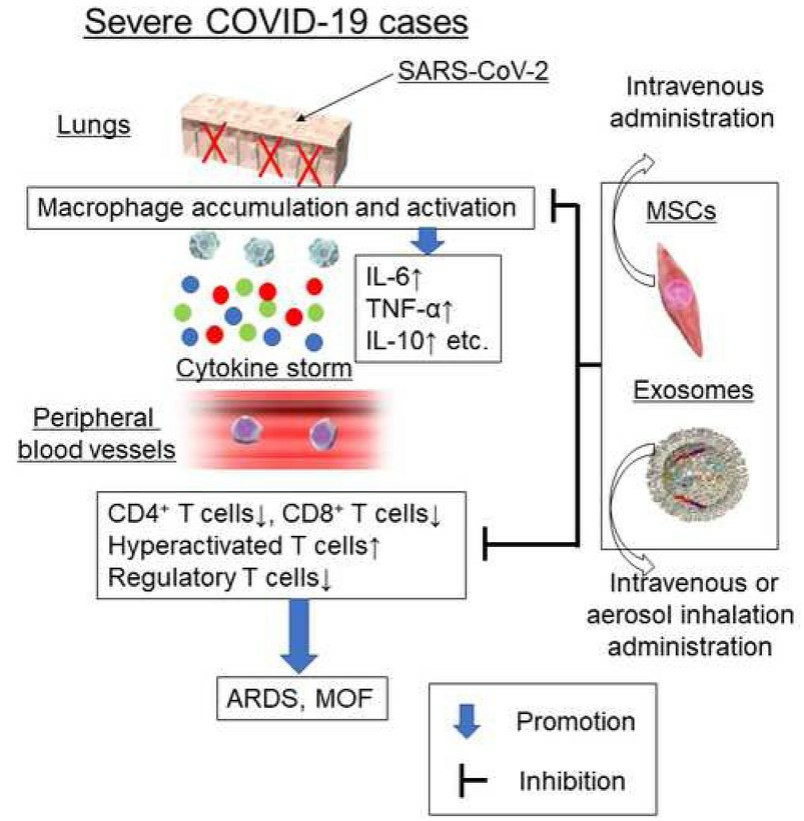 Fig.1 Putative mechanisms of MSCs and exosome therapy in severe COVID-19 cases. (Tsuchiya, 2020)
Fig.1 Putative mechanisms of MSCs and exosome therapy in severe COVID-19 cases. (Tsuchiya, 2020)
MSCs-derived exosomes have the same low immunogenicity of their source properties and address the limitation of MSCs being trapped in the lungs, facilitating studies for therapeutic applications in nebulized inhalation, and the applicability of this exosome therapy against COVID-19 has now attracted the attention and interest of many researchers. miR-21-5p delivered by MSCs-exosome alleviates oxidative stress-induced lung epithelial cell death, while the loaded growth factors FGF2, miR-145 have a significant impact on lung epithelial cell proliferation, lung tissue repair and development are important. Cytokine storm is a typical pathological feature of virus-induced immune dysregulation, and MSCs-exosome can promote the expression of immunosuppressive factor IL-10 and transforming growth factor β, thus regulating the function of infiltrating lung dendritic cells and alleviating the damage caused by excessive immune response. Furthermore, in assessing the effects of MSCs-exosome on an endotoxin-induced acute lung injury model in mice, it was found that exosomes restored normal clearance of alveolar fluid and reduced the permeability of lung proteins and the degree of pulmonary edema, which may be due to a CD44-dependent mechanism of internalized exosomes in damaged host cells. Thus, exosome-based immunomodulatory effects are a promising intervention for the treatment of COVID-19.
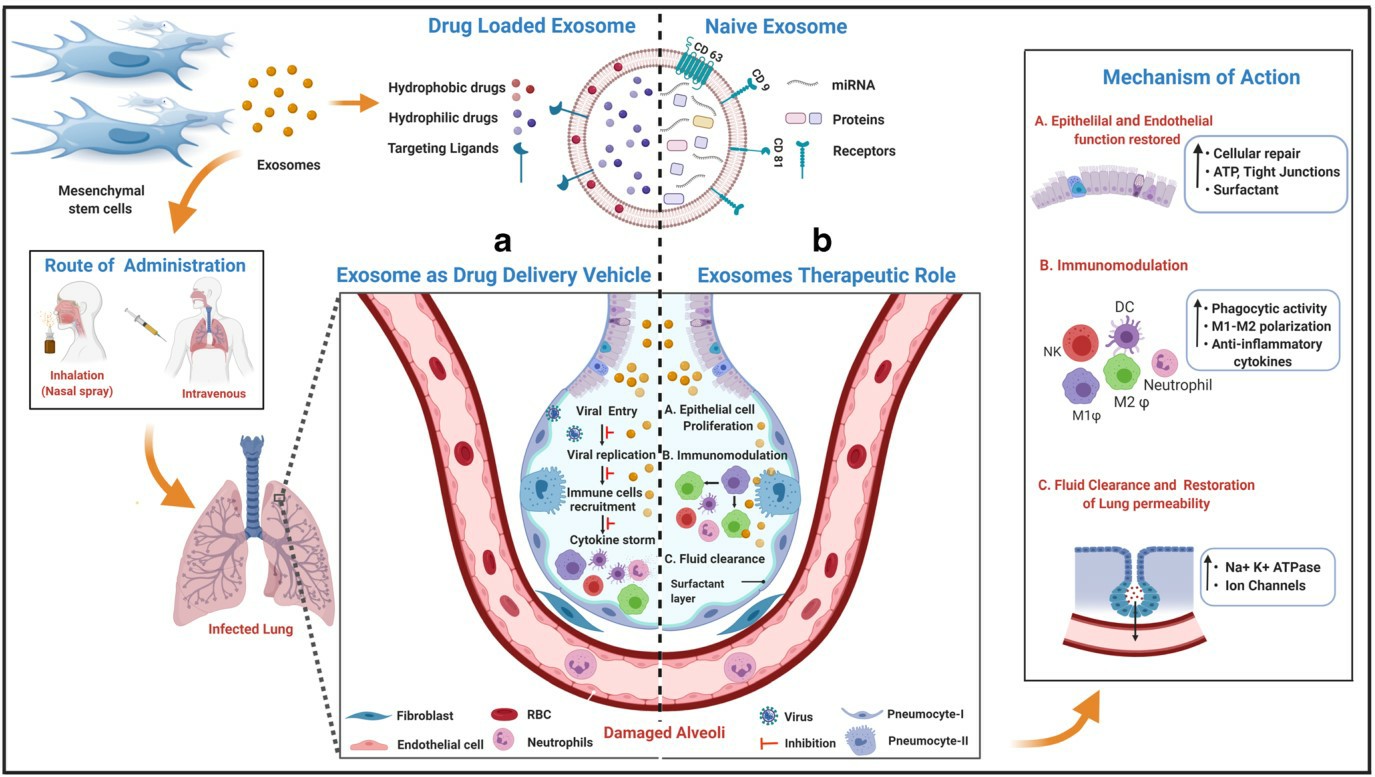 Fig.2 The potential role of MSCs derived exosomes in combating COVID-19 infection. (Pinky, 2021)
Fig.2 The potential role of MSCs derived exosomes in combating COVID-19 infection. (Pinky, 2021)
Nanometer-sized exosomes produced by all cell types can carry soluble molecules such as microRNA (miRNA), mRNA, proteins and lipids, with lipid-enclosed membranes as the main component facilitating delivery to target cells, enabling reprogramming of target cell morphology, function and fate. Compared with other lipid carriers, exosomes have tissue tropism and biological barrier penetration, which have significant advantages in maintaining biological activity and preserving their stability, protecting the encapsulated cargo from the immune and phagocytic systems during long cycles, and are considered to be excellent carriers for delivery of intended drugs to target cells. There is evidence that encapsulation of miRNAs and drugs into exosomes can act on specific molecules in infected cells to reduce local inflammation and prevent apoptosis of lung cells. The combined use of low-molecular-weight heparin and exosomes also exerted the clinical efficacy of anticoagulation in COVID-19, where lung cells underwent an anti-inflammatory, membrane stabilizing, and cytoprotective fate shift after receiving locally delivered heparin.
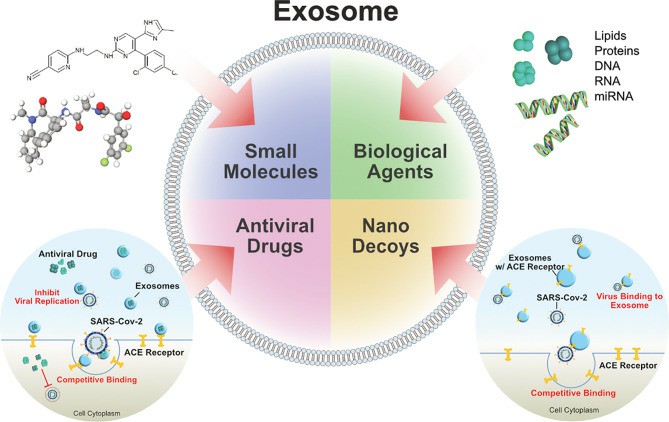 Fig.3 Exosomes as drug carrier for COVID-19. (Gurunathan, 2021)
Fig.3 Exosomes as drug carrier for COVID-19. (Gurunathan, 2021)
Exosomes are naturally occurring low-immunogenic nanovesicles with the same or similar properties as their source, which can deliver cytokines, non-coding RNAs, lipids and proteins between cells and play an important role in the performance and regulation of immune function. It has been demonstrated that exosomes have a worthwhile potential for in-depth research in disease diagnosis and treatment. Creative Biolabs can provide customers with exosome profiling services and products related to COVID-19, please feel free to contact us for your needs.
References
-
Tsuchiya, A.; et al. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm Regen. 2020, 40: 14.
-
Pinky.; et al. Mesenchymal stem cell derived exosomes: a nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev Rep. 2021, 17(1): 33-43.
-
Gurunathan, S.; et al. Diverse effects of exosomes on COVID-19: a perspective of progress from transmission to therapeutic developments. Front Immunol. 2021, 12: 716407.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Putative mechanisms of MSCs and exosome therapy in severe COVID-19 cases. (Tsuchiya, 2020)
Fig.1 Putative mechanisms of MSCs and exosome therapy in severe COVID-19 cases. (Tsuchiya, 2020)
 Fig.2 The potential role of MSCs derived exosomes in combating COVID-19 infection. (Pinky, 2021)
Fig.2 The potential role of MSCs derived exosomes in combating COVID-19 infection. (Pinky, 2021)
 Fig.3 Exosomes as drug carrier for COVID-19. (Gurunathan, 2021)
Fig.3 Exosomes as drug carrier for COVID-19. (Gurunathan, 2021)








