Western Blot (WB) Protocol
WB plays an important role in analyzing the expression of specific proteins in cell or tissue extracts. The technique relies on specific antibodies to recognize proteins separated by size through gel electrophoresis. The success of WB is related to many factors, and the success of WB is the basis for the smooth progress of many projects. For this reason, we carefully develop antibodies and provide optimized protocols as well as reference information to keep your western blots running smoothly.
Principle of WB
WB is a technique based on specific antibodies to identify proteins separated by gel electrophoresis. Charged proteins can migrate in polyacrylamide gels under the action of an electric field. Applying an electric current induces migration of proteins from the gel to the adjacent membrane. The membrane will be treated with target antibody and visualized using the secondary antibody and detection reagent.
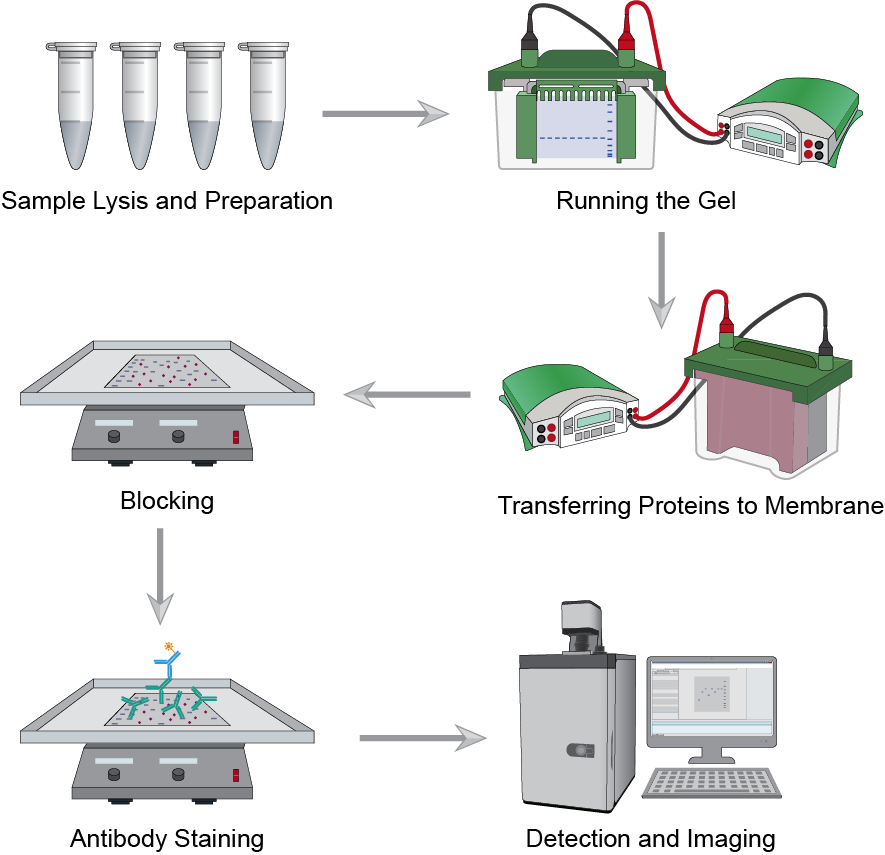 Fig.1 The workflow of western blot assay.
Fig.1 The workflow of western blot assay.
WB Protocol
- Sample preparation
- Wash the cells to be collected in the dish with pre-chilled PBS.
- Add ice-cold NP40 Cell Lysis Buffer (RIPA Lysis Buffer: 25mM Tris-HCl pH7.5, 150mM NaCl, 1% NP-40, 1mM EDTA pH8.0. Add fresh: 1 mM PMSF, 1 mM Na₃VO₄, and 1 X Protease Inhibitor Cocktail).
- Place the buffer sample solution on ice for 30 minutes to fully lyse the cells, and gently invert and mix every 10 minutes.
- Centrifuge the lysate at 12,000g in a pre-cooled centrifuge for 15 minutes.
- After low-temperature centrifugation, transfer the supernatant to a new tube and quantify the protein concentration by BCA assay.
- Determine the protein concentration for each cell lysate and add suitable volume 2 X SDS sample buffer.
- To reduce sample denaturation, boil each cell lysate in sample buffer at 100 °C for 5 min. Fresh samples are recommended, but samples can be stored at -80 °C for short periods of time.
- Electrophoresis and protein transferring
- Load same amounts of protein into each well of the SDS-PAGE gel, along with a molecular weight marker. In general, load 20-30 μg of total protein from cell lysate.
- Run the gel for 1.5h at 100 V until the dye reaches the bottom of the gel.
- Soak the gel in protein transfer buffer. Prepare the same nitrocellulose membrane as the gel transfer area. Assemble the electroblotting box according to the instructions and place the electrodes in the blotting unit.
- Transfer in Tris-Glycine transfer buffer at 100 V for 1 h at constant current.
- After transfer, remove the membrane from the blotting box and cut corners to mark the orientation of the gel. Rinse briefly with PBS.
- Membrane blocking and antibody incubations
- Wash the membrane with TBST for 3 min. Block with freshly prepared 5% nonfat dry milk for 1 h on a shaker platform.
- Incubate the membrane with a specific primary antibody diluted in TBST and 5% nonfat dried milk at the recommended dilution with gentle agitation at 4°C overnight.
- Wash three times for 5 min each with TBST.
- Incubate with secondary antibody at appropriate dilution in TBST-5% nonfat dry milk for 1 hour at room temperature.
- Wash three times again for 5 minutes each with TBST.
- Detection of proteins
For detection, prepare the developer according to the instructions. Incubate membrane with developing solution expose to x-ray film.
Creative Biolabs has accumulated rich experience in the application and improvement of WB. With advanced analytical platforms and experienced scientists, Creative Biolabs offers the most diverse portfolio of natural autoantibodies (NAA) services to facilitate your project. Please contact us in time for more details.

