Trypanosoma brucei-derived Exosome Research and Application
Trypanosoma brucei is the pathogen of African sleeping sickness, a parasitic disease spread by the tsetse fly. Through the blood-stage life cycle, Trypanosoma brucei releases exosomes, which play a pivotal role in the interaction between the parasite and its host. Creative Biolabs summarizes and forecasts the Trypanosoma brucei -derived exosome, providing clients with novel research perspectives on this pathogen.
Overview of Trypanosoma brucei
Prevalent regions: Widespread in sub-Saharan Africa.
Transmission: Spread by the bite of the tsetse fly, the primary vector in the life cycle of Trypanosoma brucei .
Biological features: Trypanosoma brucei has flagella and can form a trypanosome body; their flagellar membranes can bud to form dynamic membrane nanotubes, which eventually break to form exosomes.
Infection symptoms: They cause anemia by altering the properties of the host's red blood cells and affect the immune system, which can lead to systemic inflammation and neurological damage.
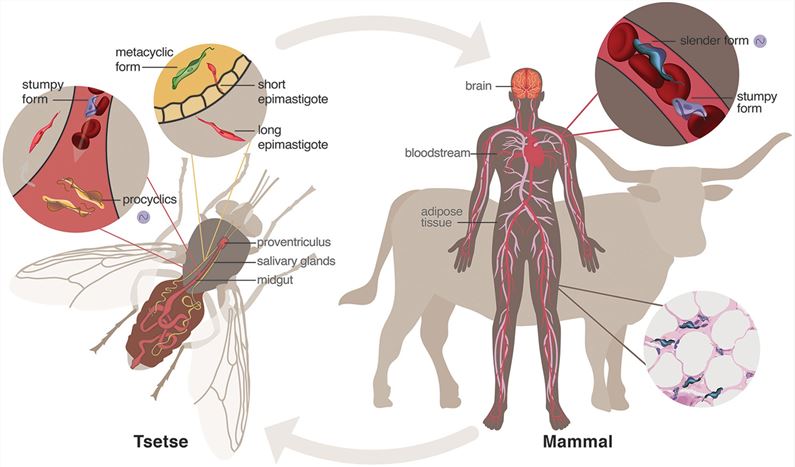 Fig.1 Life cycle of Trypanosoma brucei.1,2
Fig.1 Life cycle of Trypanosoma brucei.1,2
Processes Involved by Trypanosoma brucei -derived Exosomes
Trypanosoma brucei -derived exosomes are key secretions of the parasite cell, crucial to its survival strategy and interaction with the host.
1. Carrying information of the pathogen:
Exosomes released by Trypanosoma brucei contain molecules from the pathogen's surface, especially Variant Surface Glycoproteins (VSGs) and proteins related to the parasite's virulence, such as flagellar proteins. Exosomes from Trypanosoma rhodesiense also contain Serum-Resistance Associated (SRA) proteins, which help the parasite survive in the human host.
2. Interaction with the host's red blood cells:
Trypanosoma brucei -derived exosomes can fuse with the host's red blood cells, transferring VSGs and lipids to the surface of the red blood cells, a process that leads to changes in the properties of the red blood cell membrane, increasing their likelihood of being recognized and cleared by the host's immune system, thus leading to anemia.
3. Affecting the host's immune system:
Trypanosoma brucei -derived exosomes can manipulate the host's immune response. They affect the differentiation of macrophages, promoting the formation of M1 and M2 macrophages, as well as the expression of MHC molecules. In T cells, these exosomes induce the overexpression of CD3 and the nuclear factor FoxP3, thereby promoting the differentiation of regulatory CD4+ and CD8+ T cells. This suggests that Trypanosoma brucei -derived exosomes may play a key role in host immune regulation.
4. Inducing inflammation:
Molecules such as VSG and CpG-rich parasite DNA within Trypanosoma brucei -derived exosomes interact with receptors on host cells, leading to the production of inflammatory cytokines like TNF-α, IL-6, and IL-12 within the host.
5. Communication between parasites:
Trypanosoma brucei can continuously release exosomes or do so when RNA transcription and processing are under stress. These exosomes are absorbed by procyclic forms of the parasite in the insect host, suggesting that exosomes play a role in communication within the parasite population.
Support from Creative Biolabs
Trypanosoma brucei -derived exosomes hold vast application potential, especially in disease diagnosis and targeted therapy. These nanoscale communication tools can not only reveal disease mechanisms but may also become innovative therapeutic interventions. In this cutting-edge field, Creative Biolabs stands out with its advanced one-stop microorganism-derived exosome technology services. Please contact us to start your innovative project.
Microorganism-derived Exosome Isolation and Identification
In Vitro Functional Discovery of Microorganism-derived Exosomes
In Vivo Functional Discovery of Microorganism-derived Exosomes
References
-
Rijo-Ferreira, F.; Takahashi, JS. Sleeping sickness: a tale of two clocks. Frontiers in Cellular and Infection Microbiology. 2020, 10:525097.
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Life cycle of Trypanosoma brucei.1,2
Fig.1 Life cycle of Trypanosoma brucei.1,2








