NAA and Cancers
Autoantibodies are primarily produced by a small subset of the B cells known as B-1 cells or CD5+ B cells after the immune reactions are directed against one or more of the body's antigens (self-antigen). They may comprise proteins, nucleic acids, carbohydrates, lipids, or various combinations of these biological materials. For example, in systemic lupus and related systemic autoimmune disorders, the dominant antigens are ribonucleoproteins or deoxyribonucleoproteins. Autoantibodies may be pathogenic, disease-specific, and diagnostic, or of no apparent significance. They bind to non-foreign structures within the body and can be found in most well-defined autoimmune disorders. Low-level autoantibodies occur naturally in healthy individuals and are more common among older adults. These natural autoantibodies occur in low concentrations and have weak binding affinities. Until recently, it had been thought that high-affinity autoantibodies were only associated with autoimmune conditions. However, there is increasing evidence that these autoantibodies are also involved in chronic malignancies. Several mechanisms have been proposed to produce autoantibodies in cancer including host-immune reactions to tumor-associated antigens (TAAs), antigenic stimulation because of the destruction of malignant cells, or immune dysregulation induced by the neoplastic process.
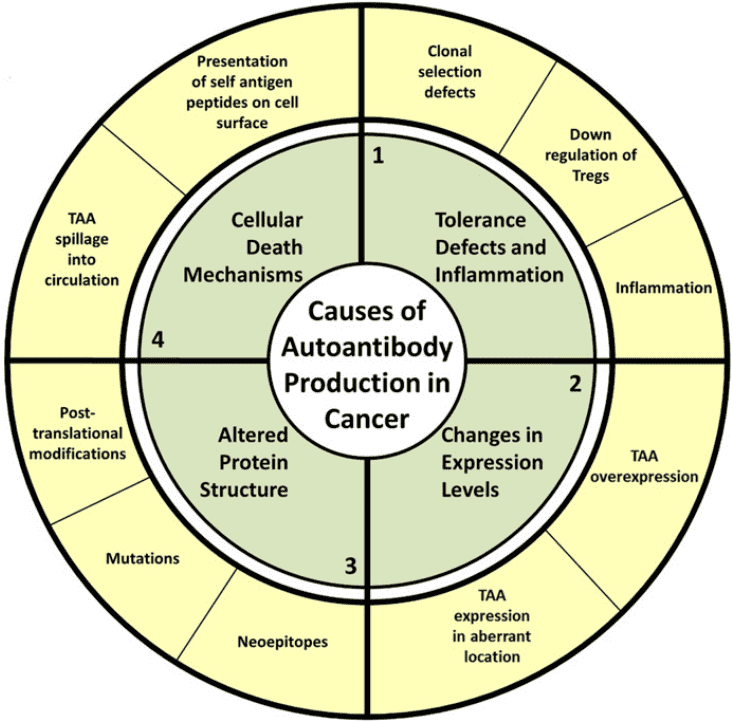 Fig.1 Proposed causes of autoantibody production in cancer.1
Fig.1 Proposed causes of autoantibody production in cancer.1
The Role of Autoantibodies in Cancer
- Early detection
- High stability
- Non-invasive marker
Autoantibodies are primarily produced by a small subset of the B cells known as B-1 cells or CD5+ B cells after the immune reactions are directed against one or more of the body's antigens (self-antigen). They may comprise proteins, nucleic acids, carbohydrates, lipids, or various combinations of these biological materials. For example, in systemic lupus and related systemic autoimmune disorders, the dominant antigens are ribonucleoproteins or deoxyribonucleoproteins. Autoantibodies may be pathogenic, disease-specific, and diagnostic, or of no apparent significance. They bind to non-foreign structures within the body and can be found in most well-defined autoimmune disorders. Low-level autoantibodies occur naturally in healthy individuals and are more common among older adults. These natural autoantibodies occur in low concentrations and have weak binding affinities. Until recently, it had been thought that high-affinity autoantibodies were only associated with autoimmune conditions. However, there is increasing evidence that these autoantibodies are also involved in chronic malignancies. Several mechanisms have been proposed for the production of autoantibodies in cancer including host-immune reactions to tumor-associated antigens (TAAs), antigenic stimulation because of the destruction of malignant cells, or immune dysregulation induced by the neoplastic process.
The autoantibodies are inherently stable, and tumor-associated autoantibodies can persist as long as the corresponding TAA triggers a specific humoral response as antibodies are not affected by proteolysis like other polypeptides. They remain in circulation with half-lives for up to 30 days and are more stable outside the body compared to other biomarkers. This persistence and stability of the autoantibodies in the blood sample has an advantage over other biomarkers, including TAAs themselves, which may be rapidly degraded or cleared by the body. For example, the extracellular protein kinase A (ECPKA) autoantibody, a universal cancer biomarker was reported to be increased in various stages of different types of cancer such as bladder, breast, colon, lung, ovarian, prostate, and pancreatic cancers when compared to healthy controls.
Current techniques for cancer detection generally require highly invasive tissue biopsy or imaging techniques such as mammography for the breast, computed tomography (CT) for lungs. These techniques are time-consuming, expensive, and associated with pain. Depending on the sensitivity of the employed screening method, these techniques are ill-suited for the early diagnosis of malignant cells. The autoantibodies against TAAs are found in the serum, plasma, saliva, cerebrospinal fluids of cancer patients where they are easily accessible for screening. The wide distribution of autoantibodies in biological fluids makes them a promising biomarker for less invasive diagnosis and more personalized monitoring of diseases. Also, the sample collection is more simplified as a result of the long half-life, i.e., seven days of the autoantibodies that minimize the hourly fluctuations.
 Fig.2 Flowchart for identifying and detecting autoantibody biomarkers in breast cancer utilizing antigen arrays and ELISA.2
Fig.2 Flowchart for identifying and detecting autoantibody biomarkers in breast cancer utilizing antigen arrays and ELISA.2
Services at Creative Biolabs
With manifold advantages in cancer research, autoantibodies have absorbed many researchers devoted to relevant studies. Focusing on autoantibodies over years, Creative Biolabs has accumulated extensive experience from practice. Based on our expertise and strong foundation, we have established a comprehensive technology platform providing a variety of autoantibody relevant services and products including but not limited to:
- NAA Detection
- NAA Profiling
- NAA Affinity Measurement
- NAA Epitope Mapping
- NAA Paratope Mapping
- NAA Detection Kits
If you are also focusing on autoantibody research, or you have any other questions about our services, please feel free to contact us for more information.
References
- Zaenker, Pauline, Elin Solomonovna Gray, and Melanie Ruth Ziman. "Autoantibody production in cancer—the humoral immune response toward autologous antigens in cancer patients." Autoimmunity reviews 15.5 (2016): 477-483.
- Qiu, Jingyi, et al. "Autoantibodies as potential biomarkers in breast cancer." Biosensors 8.3 (2018): 67.
Related Services:
- NAA and Pulmonary Diseases
- NAA and Nephropathy
- NAA and Hematological Diseases
- NAA and Endocrine Diseases
- NAA and Cardiovascular Diseases
- NAA and Neurological Diseases
- NAA and Infectious Diseases
- NAA and Autoimmune Disorders

