Based on the extensive experience and advanced technical platforms, Creative Biolabs offers customized, one-stop ECIA™ cellular analysis services designed to accelerate your immune monitoring or epitope discovery projects. We carry out the cellular assays with optimized and validated protocols, which are time-saving and cost-efficient. Our experienced scientists could deliver accurate reports and overcome the pitfalls of variability. With our unique high-throughput approach, hundreds of samples can be processed in a single program, allowing you to focus on your critical work and project planning.
Upon receiving your inquiry, our experts will engage themselves with your project quickly, design suitable cell-based analysis protocols, come up with a complete quotation including experimental design, cell preparation and shipping, details of the assay protocols, and the final report. Customers can order a single cellular analysis service or any other service from the whole epitope discovery system.
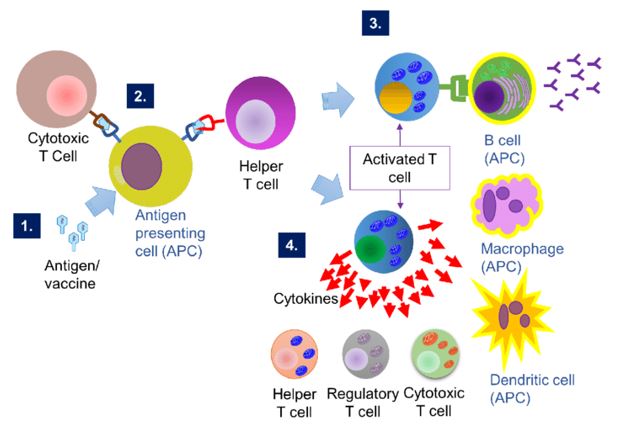 Fig.1 Cell-mediated immunity. (Wen, 2017)
Fig.1 Cell-mediated immunity. (Wen, 2017)
HLA tissue typing service is consisted of a series of systematically integrated assays to help customers acquire complicated HLA allele information for both academic research and clinical application.
We provide up-to-date HLA sequencing services and use the PCR-SSO/SSP strategies, which could meet specific requirements in sequencing resolution, time, budget, and research objectives and perfectly maintain a balance among the assay quality, time and cost. Furthermore, modules based on the Next-Generation Sequencing (NGS) are ready for a more precise analysis.
This assay can identify and enumerate the number of ASC and those secreting antibodies to a specific antigen in samples with high sensitivity at the cellular level. On the other hand, this platform can also be utilized for infection detection, drug therapy, vaccination, and autoimmunity.
The optimized ELISpot assay measures the magnitude and quality of T cells immunity through detecting antigen-specificity T cells that involve in the secretion of cytokines and other utility. This process is much more reproducible and sensitive than other assays, such as cytokine measurements in detecting low-frequency antigen-specific CD4+ and CD8+ T cells.
Intracellular cytokine staining is achieved by blocking the transfer of protein-mediated by the Golgi apparatus, by the use of in vitro polyclonal activators or specific antigen-stimulated cells and cytokine transmembrane secretion blocker, allowing the accumulation of cytokines in the cytoplasm.
This protocol can display the character of single-cell and the specificity of cell groups, which could directly reflect in vivo cell state and the level of protein in the cytoplasm.
Carboxyfluorescein succinimidyl ester (CFSE) can easily penetrate the cell membrane and covalently bind to the intracellular protein in the living cells. It will release green fluorescence after hydrolysis. The labeled cells can be observed for weeks in vivo so that they are normally utilized in the experiments of cell test and cell activity observation using fluorescent electron microscopy or confocal.
In animal research, the interaction between host and pathogen has attracted more and more attention. There has been some progress in the study of T-cell immune reagents for many animals, but it is urgent to directly evaluate pathogen-specific T-cells, a rare cell population, but the most important thing is to coordinate the host's immune response to specific pathogens. At present, we provide a technology that can quantify antigen-specific cellular immunity at the single-cell level, and can monitor individual immunity in detail based on the variability related to the number of activated T cells or the type of cytokines secreted. In veterinary medicine, the detection of pathogen-specific T cells can provide diagnostic help in the case of unclear or missing serological results.
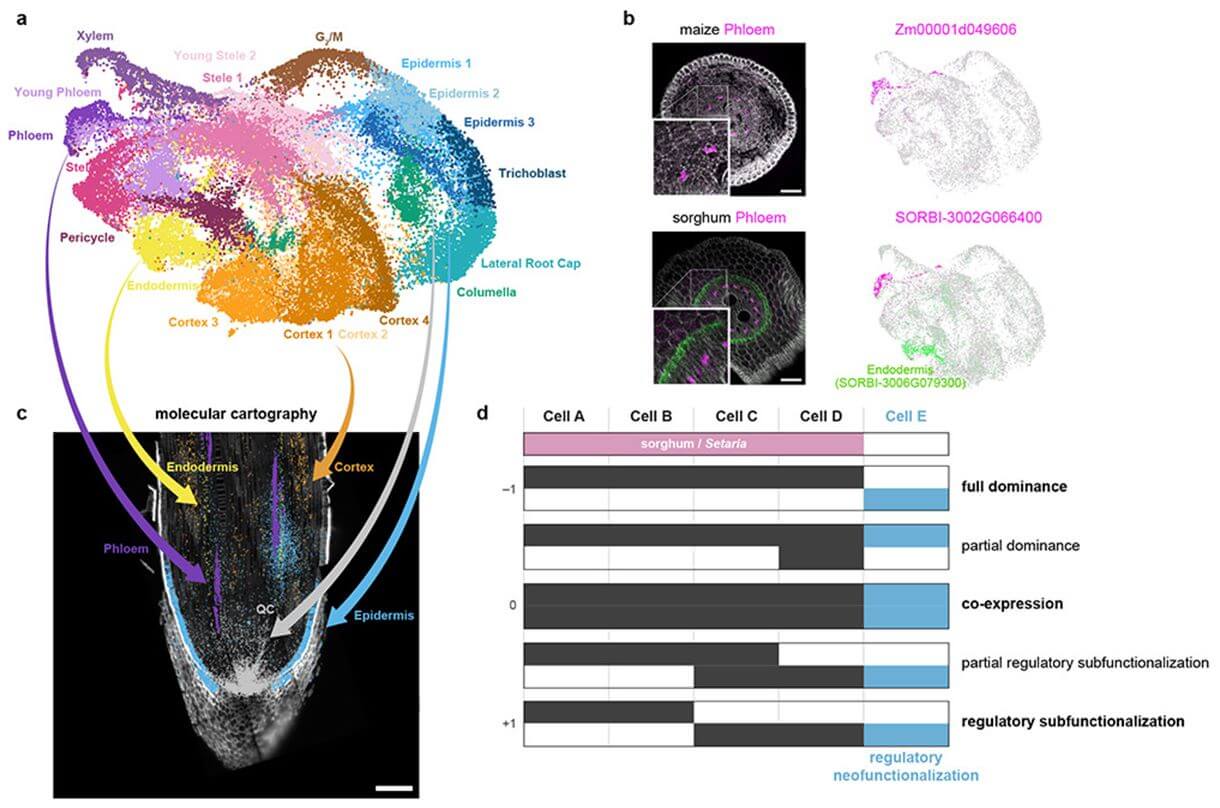 Fig. 2 Mapping cell identities from maize to sorghum and gene duplicate analysis. (Bruno Guillotin, 2023)
Fig. 2 Mapping cell identities from maize to sorghum and gene duplicate analysis. (Bruno Guillotin, 2023)
The article presents a comprehensive cellular analysis across three grass species—maize, sorghum, and Setaria—focusing on the evolution of cellular functions and the role of whole genome duplication (WGD) in diversifying gene expression across different cell types. By leveraging single-cell and single-nucleus RNA sequencing, the study identifies patterns of gene expression that have diverged among these species, particularly highlighting the effects of WGD in maize. The researchers discovered that certain cell types, especially those undergoing rapid evolutionary change, exhibit unique gene expression patterns facilitated by the WGD, suggesting a role for these genetic events in adapting to environmental pressures or agricultural selection. This method helps to pinpoint orthologous marker genes and understand the cellular basis for species-specific traits. The cellular profiling data enable a deeper understanding of how WGD influences gene expression at the cellular level, providing insights into the evolutionary trajectories of these important crop species.
Cellular analysis involves studying individual cells and their properties, particularly how immune cells recognize and respond to specific epitopes. This analysis is crucial for understanding immune system dynamics and identifying how different cells react to pathogens, allergens, or vaccines, ultimately aiding in the development of immunotherapies and vaccines.
Cellular analysis accelerates immune monitoring by providing detailed insights into the cellular mechanisms of immune responses. Techniques like flow cytometry and mass cytometry (CyTOF) allow for the high-throughput analysis of multiple markers on individual cells, enabling rapid profiling of immune cell populations and their functional states during disease progression or in response to treatment.
In epitope discovery, cellular analysis helps identify the specific parts of antigens that are recognized by T cells or antibodies. By isolating and characterizing antigen-specific cells, researchers can map the interactions between these cells and potential epitopes, facilitating the design of targeted vaccines and therapeutics.
Technologies commonly used in cellular analysis include flow cytometry, mass cytometry, single-cell RNA sequencing (scRNA-seq), and peptide-MHC multimer staining. Each of these technologies allows for the detailed examination of cell surface markers, cytokine production, gene expression profiles, and antigen specificity.
Cellular analysis contributes to personalized medicine by enabling the detailed characterization of individual immune responses. This allows for the development of personalized immunotherapies and vaccines based on a person's specific immune landscape and disease profile, potentially improving treatment efficacy and reducing adverse effects.
Cellular analysis is critical for detecting minimal residual disease in conditions like cancer. Techniques such as flow cytometry and scRNA-seq can identify rare populations of disease cells that remain after treatment, providing crucial information for assessing treatment success and guiding subsequent therapeutic decisions.
Cellular analysis help scientists directly observe how immune cells respond to vaccine candidates. Understanding these responses at a cellular level helps in optimizing vaccine components to enhance immunogenicity and efficacy, and in identifying potential adverse immunological reactions before clinical trials.
Recent advancements enhancing cellular analysis techniques include the development of multi-omic technologies that integrate genomic, transcriptomic, and proteomic data from single cells, and improvements in computational methods for analyzing large datasets. These advancements provide a more comprehensive understanding of cellular behaviors and interactions.
Cellular analysis informs the adjustment of immunotherapies by providing detailed feedback on how immune cells react to these treatments. By analyzing changes in immune cell populations and functions in response to therapy, clinicians can tailor treatment plans to maximize effectiveness and minimize adverse effects, ensuring optimal patient outcomes.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
