β2M-Expressed Exosome Modification Service
Tumor-associated macrophages are central to the network of immunosuppressive cells and cytokines and play a crucial role in tumor immune evasion. In the process of tumor immune evasion, highly expressed β2-microglobulin (β2M) is involved in the escape of tumor cells. However, β2M is not only a promising target for tumor therapy but also a protein with anti-phagocytic function. Due to their natural material transport properties, inherent long-term circulation ability, and excellent biocompatibility, exosomes have great potential to be drug delivery vehicles, suitable for delivering various small molecule drugs, proteins, nucleic acids, and other therapeutic substances. Although modification of targeting molecules on the surface of exosome membranes can increase the accumulation of these exosomes at the target site. However, some of these engineered exosomes were found to disappear in the body's circulation, in part because macrophages in the body's immune system see these exosomes as dangerous and clear them. It is gratifying that the preliminarily mature exosome modification technology can endow exosomes with anti-phagocytic ability. Creative Biolabs can construct β2M-expressed exosomes for customers through exosome production pathway modification or chemical modification, thereby further amplifying the strength of exosomes as a drug delivery system.
β2M Overview
β2M is a component of the light chain structure of the major histocompatibility complex I (MHC I) on the cell membrane. The amino acid sequence of β2M is highly conserved, with little difference among different species. Therefore, β2M of different species can replace each other. The role of β2M is mainly to stabilize MHC I and enable them to be effectively expressed on the cell surface. Some tumor cells highly express β2M, which can bind to immunoglobulin-like receptor B (LILRB) on macrophages to inhibit phagocytosis, resulting in loss of immune surveillance. In addition, in patients with normal or high expression of MHC I on tumor cells, drugs targeting the MHC I/LILRB1 axis may promote anti-tumor immune responses and exert synergistic effects with drugs targeting the CD47/SIRPα axis. Therefore, β2M is a protein with anti-phagocytic properties. Researchers have proposed that β2M can be modified on the surface of exosomes to increase the half-life of exosomes in the circulation system.
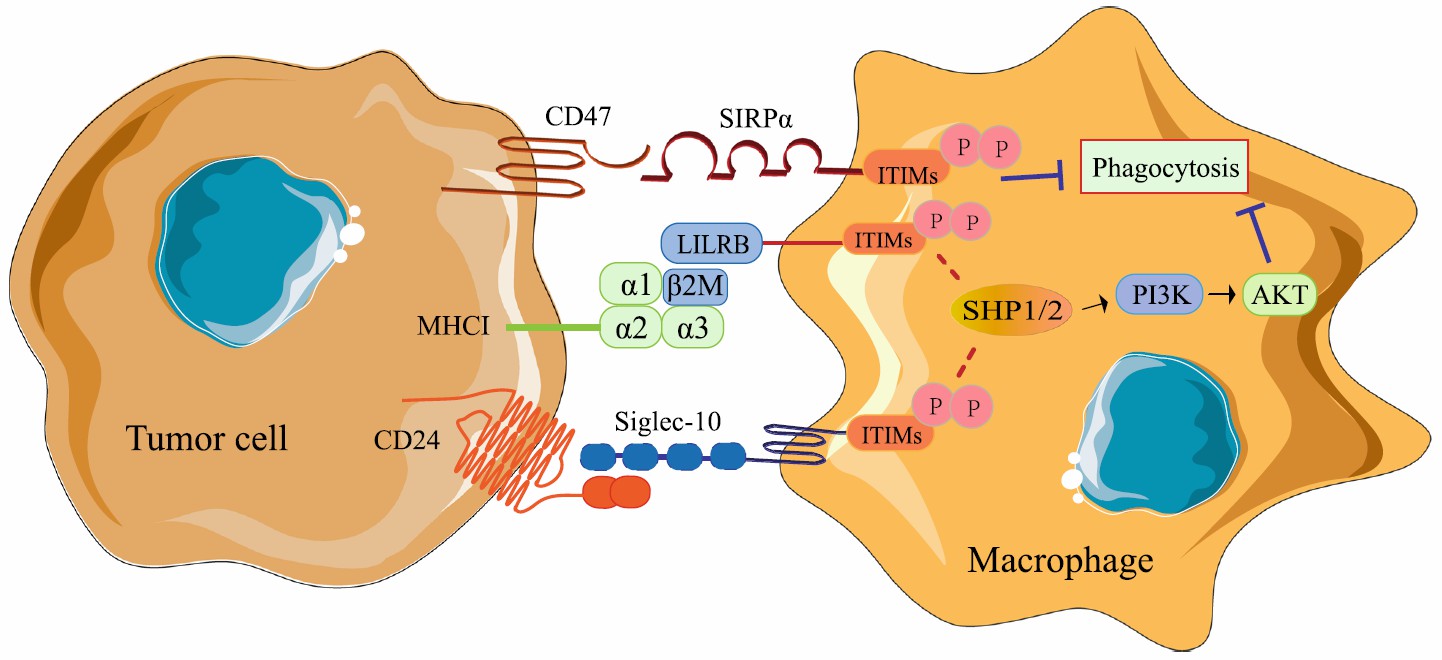 Fig.1 The mechanisms of macrophages participating in tumor antigen recognition disorder.1,2
Fig.1 The mechanisms of macrophages participating in tumor antigen recognition disorder.1,2
β2M-Modified Exosome Service at Creative Biolabs
At present, the preliminary and mature exosome modification technology includes the modification of exosome membrane protein based on the transformation of the exosome production pathway and the modification of exosome phospholipid molecules or exosome membrane protein based on chemical methods. The former technology can achieve modification by co-expressing β2M with exosome membrane proteins in donor cells. The latter technology can package β2M into molecules with lipophilic properties or molecules with affinity. These molecules can be attached to the exosome membrane through hydrophobic interactions or binding forces. At Creative Biolabs, we can customize the most suitable β2M-modified exosome solution and provide the most efficient technical services for customers according to the source of exosomes and the goals of the customers' project.
Creative Biolabs, a biomedical high-tech enterprise focusing on exosome development, has been continuously launching efficient exosome research tools around customer needs. We have laid out three major business segments, namely exosome engineering, exosome sequencing, and exosome function research in vivo and in vitro, to help customers tap the potential of exosomes. If you want to enhance the in vivo retention of drug-loaded exosomes, please feel free to contact us.
References
-
Qiu, Y.; et al. Next frontier in tumor immunotherapy: macrophage-mediated immune evasion. Biomarker Research. 2021, 9(1):72.
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 The mechanisms of macrophages participating in tumor antigen recognition disorder.1,2
Fig.1 The mechanisms of macrophages participating in tumor antigen recognition disorder.1,2








