Therapeutic Exosomes for Cutaneous T Cell Lymphoma
Exosomes have demonstrated potential as cell-derived, low-immunogenicity delivery vehicles in the treatment of cutaneous T-cell lymphoma (CTCL). Creative Biolabs has the most specialized technical expertise in exosome research and a state-of-the-art research platform to provide CTCL-related exosome research services to meet our clients' customized needs.
Overview of CTCL
CTCL is a group of complex heterogeneous malignancies caused by clonal reproduction of helper/memory T lymphocytes that originate from skin lesions and can accumulate in bone marrow, lymph nodes and visceral organs, among others, while mycosis fungoides (MF) and Sézary syndrome (SS) are two of its common subtypes. In the early stages, CTCL has a more similar pathogenetic profile to benign cutaneous inflammatory dermatoses, creating difficulties for clinical and histopathological diagnosis, with up to 5 years of pathological progression and multiple skin biopsies to demonstrate clear diagnostic markers. Until now, the lack of reliable biomarkers has hampered the study of CTCL pathogenesis, and various factors such as abnormal molecular genetic pathogenesis, immune abnormalities and disorders, viral infections and environment have been considered as possible lesion drivers. Among them, abnormal immune regulation plays a non-negligible role in causing CTCL pathogenesis, while the development of several cytokine-related clinical trial drugs such as interferons and interleukins emphasizes this etiology.
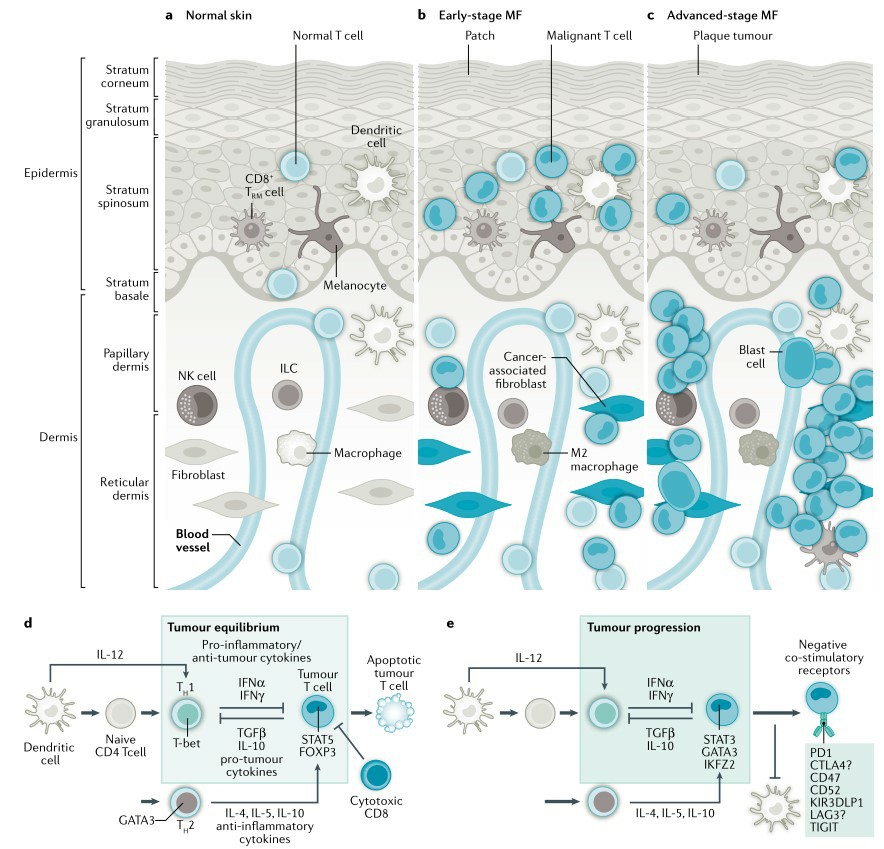 Fig.1 Pathogenesis of mycosis fungoides. (Dummer, 2021)
Fig.1 Pathogenesis of mycosis fungoides. (Dummer, 2021)
Therapeutic Exosomes for CTCL
Treatment regimens for CTCL are developed according to the short or long term of the treatment goal and include skin-specific therapies, such as topical steroids or phototherapy, and systemic therapies, such as monoclonal antibodies or chemotherapy. These treatments, however, are not sufficient to completely ablate the tumor and carry the risk of impairing immune function and producing toxic side effects. Currently, hematopoietic stem cell transplantation is the only promising curative treatment. New therapeutic approaches are still in clinical trials, such as vaccine therapy, chimeric antigen receptor T cells or immunomodulatory drugs. However, the ideal targeted CTCL delivery platform needs to be able to accumulate highly in tumor tissue, penetrate deeply and be taken up by CTCL in large quantities to exert efficient tumor killing effects. Cell-derived exosomes have natural advantages in terms of biostability, biocompatibility, low immunogenicity and low toxicity, and exosomal proteins such as CD81 and CD9 confer the ability to degrade the extracellular matrix and penetrate deeply into tumors, enabling exosomes to exhibit excellent cellular uptake and tumor targeting capabilities as delivery vehicles in vivo.
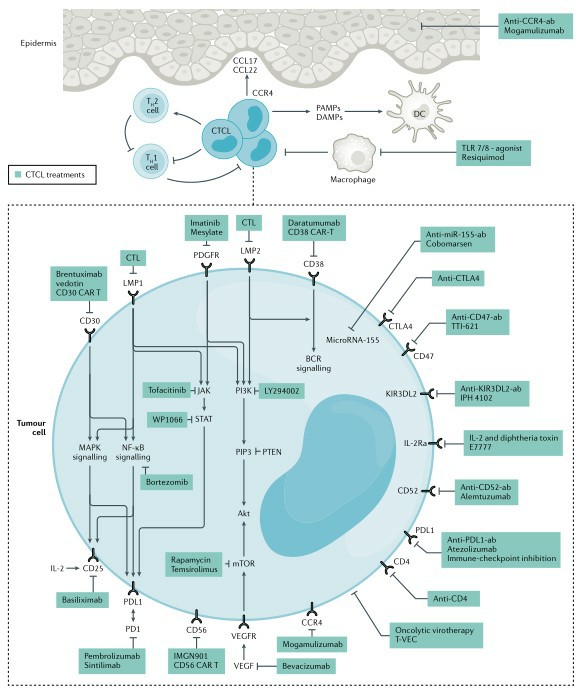 Fig.2 Targets for future directions for CTCL therapy. (Dummer, 2021)
Fig.2 Targets for future directions for CTCL therapy. (Dummer, 2021)
Malignant T cells in CTCL secrete excessive amounts of cytokines IL-4, IL-5 and IL-10, accompanied by an abnormal progressive decrease in IL-12 and IFN-y secretion. Therapeutic trials applying IL-12 induced production of T helper cell-type cytokines, enhanced cytotoxicity of natural killer cells, and stimulated IFN-y production, leading to remission of CTCL. However, this strategy still has the potential for further relapse, which may be caused by an irrational route of administration. The efficacy of intradermal injections over systemic administration is significant, thus emphasizing the need for highly targeted delivery strategies. Pipeline phase I clinical trials have demonstrated that exosomal drug strategies carrying IL-12 exert potent local pharmacological effects by precisely targeting CTCL, reducing systemic exposure to IL-12 and significantly improving pharmacodynamic activity and antitumor immune response. Thereafter, exosome delivery will continue to be investigated in tumors that are clinically responsive to IL-12 as a monotherapy, such as melanoma, Merkel cell carcinoma, triple negative breast cancer, and Kaposi's sarcoma, among many others.
Creative Biolabs offers a wide range of services related to exosome research and development, as well as custom drug development services to meet your needs for tumors such as CTCL. Please contact us with your interest.
Reference
-
Dummer, R.; et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021, 7(1): 61.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Pathogenesis of mycosis fungoides. (Dummer, 2021)
Fig.1 Pathogenesis of mycosis fungoides. (Dummer, 2021)
 Fig.2 Targets for future directions for CTCL therapy. (Dummer, 2021)
Fig.2 Targets for future directions for CTCL therapy. (Dummer, 2021)








