Therapeutic Exosomes for Hematologic Tumor
Due to their naturally inherent biological, functional and tolerability characteristics, exosomes can provide selective delivery of potent drug molecules and trigger desired pharmacological effects at desired tissue and cellular sites. Creative Biolabs has many years of proven research expertise in the biotechnology market and can provide clients with rapid and extensive exosome research services and functional efficacy assessments.
Hematologic Tumor and Exosome
Hematologic tumor, including various types of leukemia, lymphoma and multiple myeloma (MM), is caused by abnormal differentiation and proliferation of blood cells. Leukemia is a malignant disease in which malignantly proliferating leukemic cells in hematopoietic organs such as bone marrow, spleen and liver enter the peripheral blood and adsorb normal blood cells and cause infiltration of tissues and organs throughout the body. Lymphoma is a malignant disease in which tumorigenic proliferation of lymphocytes or tissues can be seen histologically. MM is caused by clonal abnormal proliferation of plasma cells in the bone marrow that secrete immunoglobulins and their fragments and damage related tissues and organs, with the hallmark pathological features being osteolytic lesions and pathological fractures. Although the specific etiology of different species of hematologic tumors varies, exosomes, as carriers of intercellular transmission of molecules and genetic material, maintain communication between bone marrow luminal cells and cells in distant tissue sites within the hematopoietic system. Hematologic tumor-derived exosomes (TEX) maintain tumorigenesis and spread through paracrine signaling, reprogramming the tumor microenvironment, thereby interfering with and suppressing antitumor immunity and mediating drug resistance. Thus, TEX plays a non-negligible mediating role in the malignant transformation of hematological tumors and has become a promising biomarker and potential therapeutic target for the study of tumor pathological processes. Its biological mechanism of mediating information transfer and evasion of the host immune system during disease progression is one of the current research hotspots.
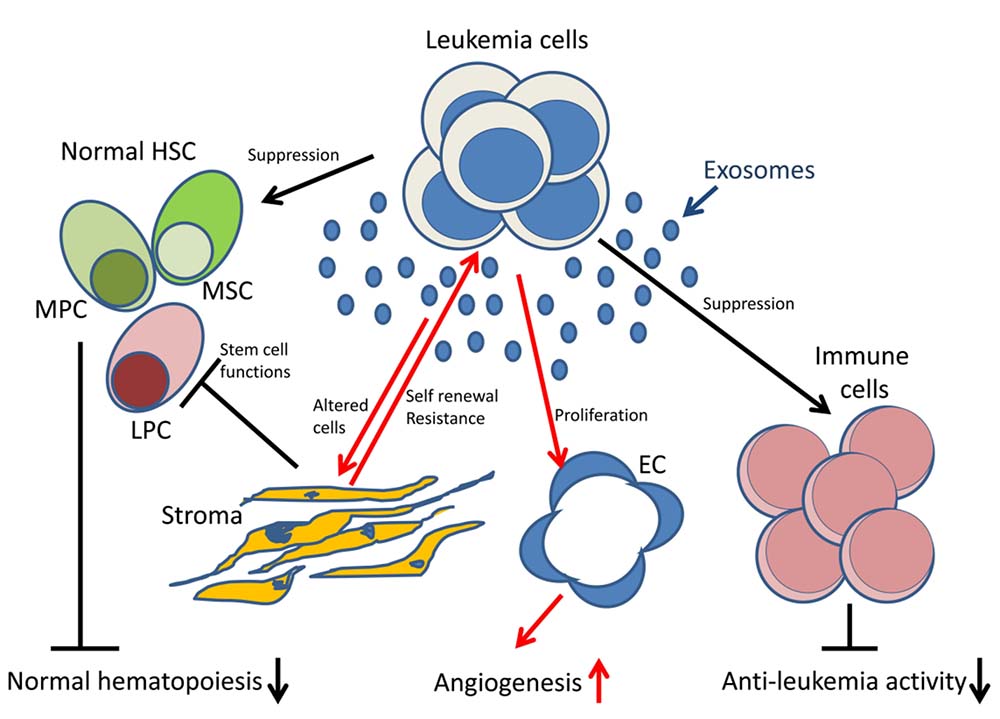 Fig.1 Leukemia-derived exosomes in the bone marrow environment. (Boyiadzis & Whiteside, 2017)
Fig.1 Leukemia-derived exosomes in the bone marrow environment. (Boyiadzis & Whiteside, 2017)
Therapeutic Exosomes for Hematologic Tumor
As membranous vesicles naturally derived from early endosomes, exosomes are promising as vehicles for the delivery of drugs, genes and other therapeutic molecules. By carrying components such as cytokines, RNA, growth factors, proteins and lipids, exosomes can modulate the metabolism of cancer cells and the function of immune effectors, thereby shifting the tumor microenvironment (TME) from a tumor-friendly to an anti-tumor environment. Natural killer (NK) cells have a significant antitumor effect, but the complex and variable TME greatly limits the ability of NK cells to penetrate deeper into the tumor. It was shown that exosomes produced from IL-2 or IL-5 stimulated cultured NK cells contain several important cytotoxic factors, including IFN-γ, lymphocyte function-associated antigen (LFA-1), DNAX helper molecule-1 (DNAM1), and programmed cell death protein (PD-1), which significantly killed leukemic tumor cell lines K562 and NALM-18. CAR-T cell-derived exosomes present CD19 CAR to CD19-positive B-cell tumors with cytotoxic and enhanced programmed death genes in CD19 leukemia B cells, reducing the risk of apoptosis of CD19-negative cells due to excessive cytokine release. Furthermore, manipulation of exosomes to deliver lymphoma-specific antigens to dendritic cells (DCs) and reconstitution of antigen-presenting capacity of DCs triggered effective CD4+/Th1 and CD8+/CTL-dependent hematologic tumor cell death, suggesting the possibility of developing antitumor immune vaccines based on DCs and tumor cell-derived exosomes.
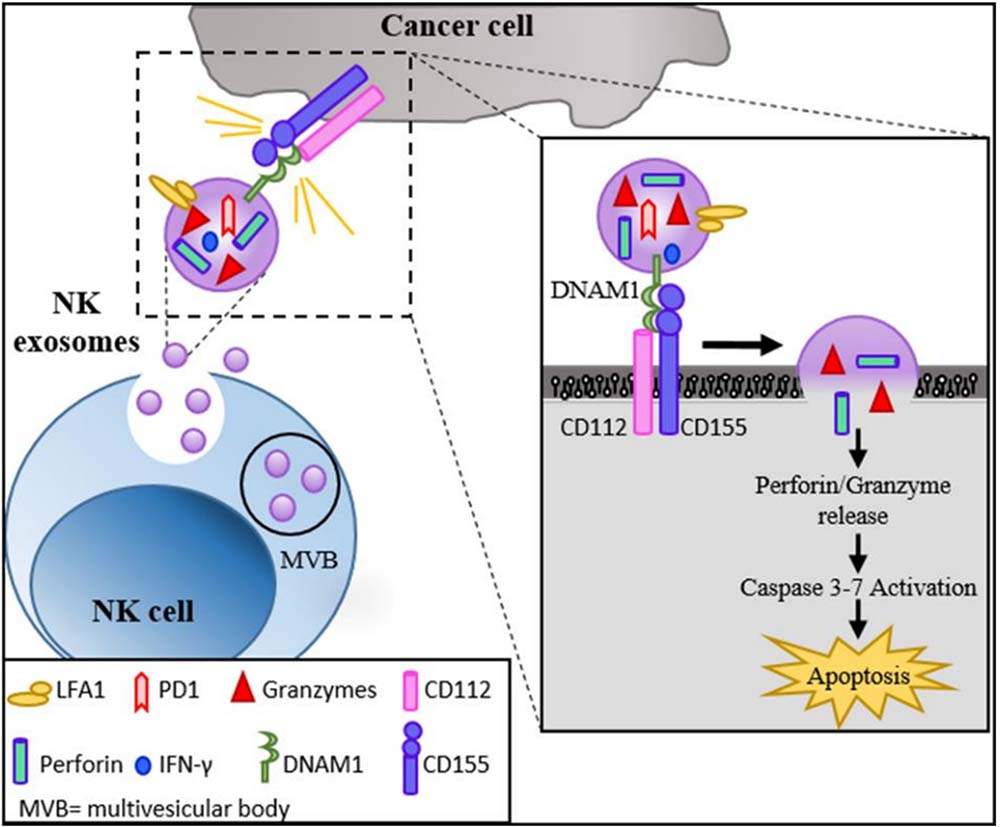 Fig.2 NK exosomes characterization and mechanism of action. (Di Pace, 2020)
Fig.2 NK exosomes characterization and mechanism of action. (Di Pace, 2020)
Whether it is exosome extraction and characterization, functional studies, or drug carriers, Creative Biolabs' experienced consultants and advanced experimental platforms can provide you with comprehensive services based on engineered exosome technology. Please feel free to contact us for more information.
References
-
Boyiadzis, M.; Whiteside, T.L. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017, 31(6): 1259-1268.
-
Di Pace, A.L.; et al. Characterization of human NK cell-derived exosomes: Role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers (Basel). 2020, 12(3): 661.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Leukemia-derived exosomes in the bone marrow environment. (Boyiadzis & Whiteside, 2017)
Fig.1 Leukemia-derived exosomes in the bone marrow environment. (Boyiadzis & Whiteside, 2017)
 Fig.2 NK exosomes characterization and mechanism of action. (Di Pace, 2020)
Fig.2 NK exosomes characterization and mechanism of action. (Di Pace, 2020)








