Therapeutic Exosomes for Myeloma
Myeloma is a blood disease that remains incurable. The natural nanostructure of exosomes makes them ideal vehicles for drug delivery. Exosomes and their processed products can be made into vaccines, or carry drugs to play a potential role in the treatment of myeloma. As a leading exosome research service provider, Creative Biolabs has rich experience in exosome drug research and can provide customers with comprehensive and high-quality myeloma-related exosome drug research services.
Myeloma and Exosome
Myeloma, also known as plasmacytoma, is the second most common hematological malignancy. Myeloma is a malignant tumor formed by the proliferation and malignant transformation of plasma cells in the bone marrow. The normal function of plasma cells is to produce antibodies (also called immunoglobulins) that help the body attack and kill pathogens. Plasma cells undergo malignant transformation, often affecting multiple sites, and are also known as multiple myeloma. Myeloma patients are often accompanied by multiple osteolytic lesions, hypercalcemia, anemia, and kidney damage. BMSC-derived exosomes in the myeloma microenvironment can increase myeloma cell proliferation, migration, and drug resistance through cytokines, chemokines, integrins, and caspase family proteins. In addition, myeloma-derived exosomes also affect other types of cells in the myeloma microenvironment through some miRNAs, cytokines, chemokines, etc., increasing the number of osteoclasts, angiogenesis, and immunosuppression of Myeloid-derived suppressor cells (MDSCs).
 Fig.1 Effects of exosomes from BMSC in microenvironment on multiple myeloma cells.1,2
Fig.1 Effects of exosomes from BMSC in microenvironment on multiple myeloma cells.1,2
Therapeutic Exosomes for Myeloma
At present, autologous hematopoietic stem cell transplantation and drugs targeting tumor cells and the bone marrow microenvironment, such as immunomodulators, proteasome inhibitors, and monoclonal antibodies, have significantly prolonged the overall survival of myeloma patients. However, myeloma is currently incurable, and almost all patients relapse. Therefore, there is an urgent need to explore new myeloma treatments. As nanoscale extracellular vesicles, exosomes are small in size, can effectively avoid the phagocytosis of mononuclear macrophages, and can freely pass through the blood vessel wall and extracellular matrix. In addition, exosomes are derived from the body and have better biocompatibility, lower immunogenicity, and homing. Therefore, exosomes can be used as drug delivery vehicles to achieve targeted drug delivery in the body. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) can selectively induce tumor cell apoptosis, but its clinical application is limited due to the short half-life of artificial recombinant TRAIL in vivo. TRAIL-loaded exosomes can induce myeloma cell apoptosis and inhibit cancer progression in vivo.
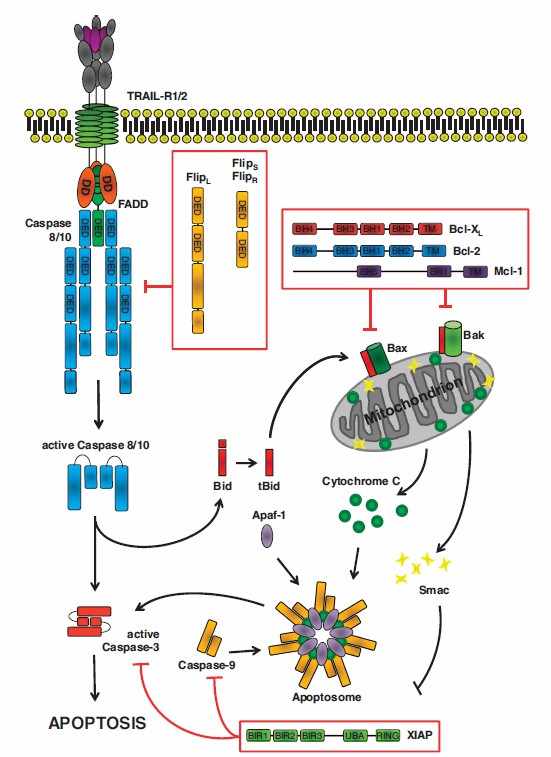 Fig.2 TRAIL-induced apoptosis.3
Fig.2 TRAIL-induced apoptosis.3
In addition, an exosomal vaccine with myeloma epitopes has been shown to induce significant cytotoxic T lymphocyte responses in vitro. This exosome vaccine is constructed by combining the source epitope of myeloma-specific antigen 1 with Dickkopf-1. Compared with the single-epitope vaccine, the exosome vaccine improves the survival rate, reduces the tumor burden, and reduces bone destruction, which is beneficial to the individualized treatment of myeloma patients.
Creative Biolabs has always adhered to a rigorous scientific research attitude and is committed to the development of exosome drug research platforms. We can provide a comprehensive and rigorous approach to your exosome drug program to help you achieve your goals. Please feel free to contact us to discuss your needs and let us explore new ways of treating myeloma.
References
-
Li, M.; et al. Potential Therapeutic Roles of Exosomes in Multiple Myeloma: A Systematic Review. Journal of Cancer. 2019, 10(24):6154-6160.
-
under Open Access license CC BY 4.0, without modification.
-
Lemke J.; et al. Getting TRAIL back on track for cancer therapy. Cell Death and Differentiation. 2014, 21(9):1350-1364.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Effects of exosomes from BMSC in microenvironment on multiple myeloma cells.1,2
Fig.1 Effects of exosomes from BMSC in microenvironment on multiple myeloma cells.1,2
 Fig.2 TRAIL-induced apoptosis.3
Fig.2 TRAIL-induced apoptosis.3








