Creative Biolabs offers professional outer retinal barrier resistance assay service to clients, facilitating the acceleration of drug development processes in the discovery model for age-related macular degeneration (AMD) based on induced pluripotent stem cells (iPSCs).
The outer retinal barrier is one of the key structures in the eye, situated between the retinal pigment epithelial cells (RPE) and the photoreceptors. It plays a crucial role in maintaining retinal environmental stability, including the transportation of nutrients, waste elimination, and prevention of harmful substances from invading the retina. The outer retinal barrier resistance assay is a method for evaluating and measuring the integrity and function of the outer retinal barrier. This test, through electrophysiological techniques, measures the transepithelial resistance (TER) of the retinal cell layer, which is used to express the resistance value density of the retinal cell layer. The value reflects the sealing degree and integrity of the barrier directly.
Using the outer retinal barrier resistance Test, we can evaluate changes in the function of the outer retinal barrier under different conditions or after specific drug treatments for our clients. By accurately comparing the functional status of the outer retinal barrier, this method helps researchers understand whether drugs can protect or restore the integrity of the barrier.
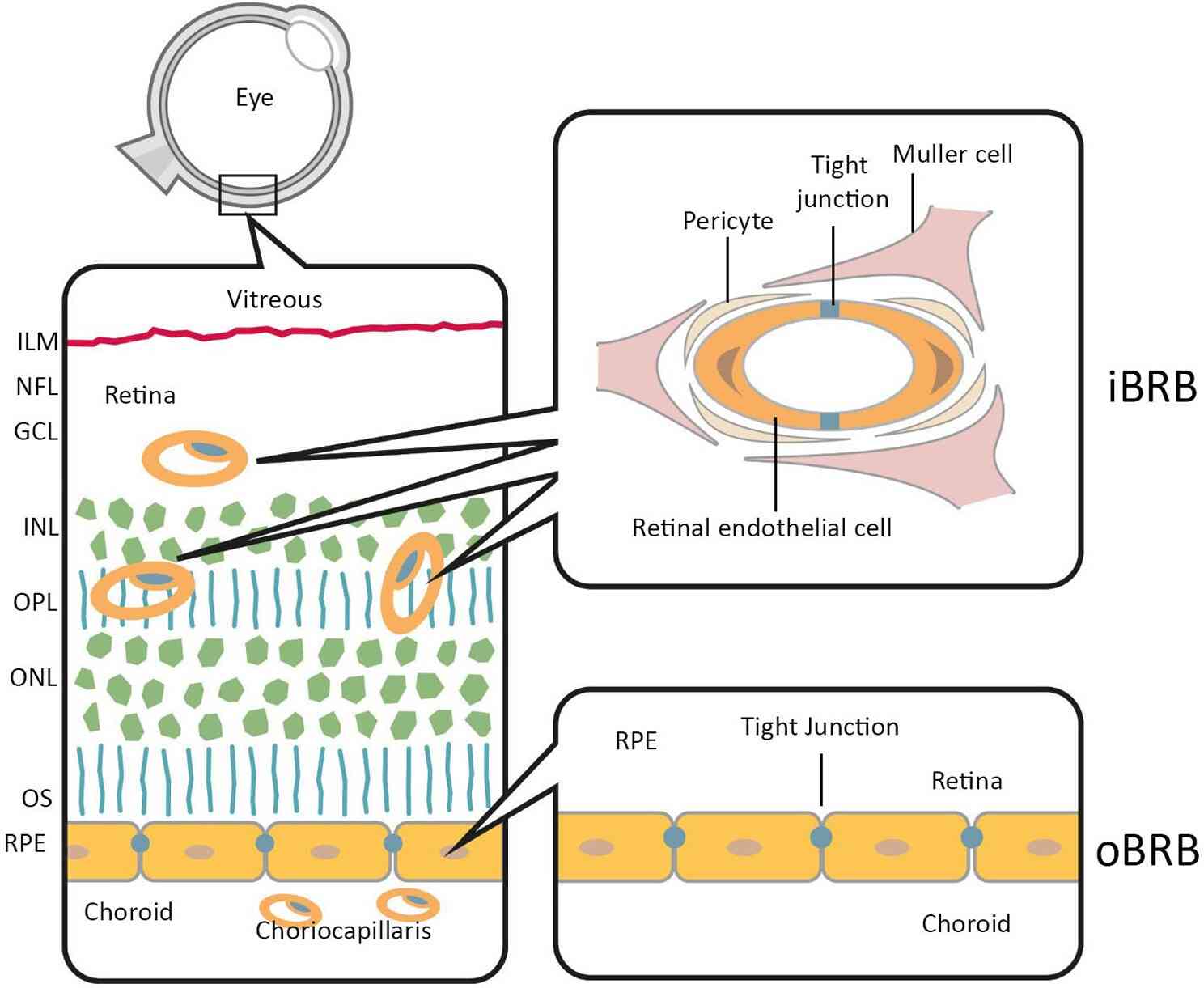 Fig.1 Anatomical localization of the inner and outer blood-retina barriers.1
Fig.1 Anatomical localization of the inner and outer blood-retina barriers.1
Using iPSC-facilitated discovery model to study AMD is an innovative research method. It leverages cells sourced from AMD patients to simulate the disease process, providing a unique and powerful tool for disease research and drug development. In this context, conducting outer retinal barrier resistance assay through this innovative AMD model to screen or evaluate drugs allows clients to assess the effects of drugs within a system highly relevant to human AMD, accelerating the exploration of treatments for AMD.
Q1: In what other research areas can the outer retinal barrier resistance test be used?
A1: This service is widely applicable to multiple research fields, including but not limited to drug development and mechanism studies for diabetic retinopathy, genetic retinal diseases, and evaluating any treatment strategies that might affect retinal health.
Q2: How long does it take to get test results?
A2: The project timeline may vary depending on specific needs, including cell culture time, drug treatment, and test duration. Typically, a complete test cycle (from cell culture to final data analysis) may take 10-12 weeks.
Q3: How are test results interpreted?
A3: Test results are usually reported in resistance values (Ω/cm²), where higher resistance values indicate stronger barrier function.
The pathogenesis of AMD includes damage to the retinal barrier. Research analyzing the effects of thrombin inhibitors has found that thrombin can reduce TER values, suggesting that thrombin participates in the pathological process of AMD by damaging the retinal barrier.
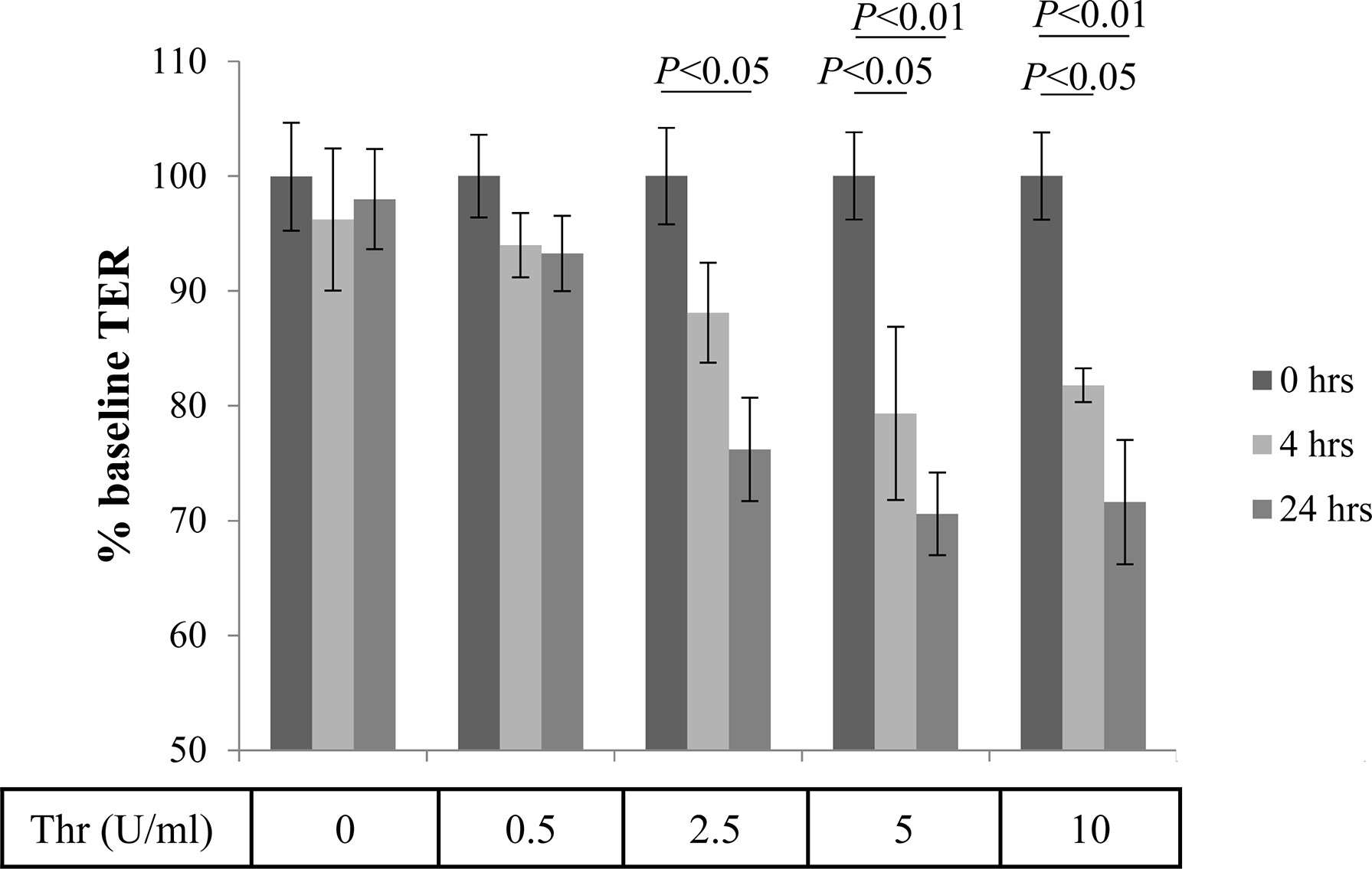 Fig.2 Thrombin-mediated effects of Transepithelial Resistance (TER).2
Fig.2 Thrombin-mediated effects of Transepithelial Resistance (TER).2
Creative Biolabs provides professional outer retinal barrier resistance assay services aimed at assisting clients in accurately assessing the effectiveness of candidate drugs in protecting the outer retinal barrier. If you have a need for this test, please feel free to contact us.
Reference
For Research Use Only. Not For Clinical Use.