Overview Service Features Published Data FAQs Scientific Resources Related Services
Creative Biolabs strives to transition iPSC technology into safe, effective and consistent therapies for various diseases and cancers effecting populations worldwide. Based on our extensive experience, now we can provide the teratoma formation assays to characterize the pluripotency of iPSCs for our customers all over the world.
Overview of Teratoma Formation Assays for iPSC
What is Teratoma Formation?
Derived from various adult somatic cell types via the introduction of four specific transcription factors: OCT3/4, SOX2, KLF4, and c-MYC, induced pluripotent stem cells (iPSCs) have the ability to differentiate into derivatives of all three germ layers and show great potential in fields ranging from regenerative medicine to disease modeling. The teratoma always means a nonmalignant tumor which comprised of a disorganized cell mixture and small tissues containing cells from all three embryonic germ-layers. With any iPSC lines, it is necessary to confirm pluripotency and characterize the cell lines before the following downstream regulation experiments. Teratoma formation is an essential criterion not only for determining the pluripotency but also for assessing the tumorigenic potential of human iPSCs. Now Creative Biolabs has built a standard protocol for teratoma assay based on subcutaneous co-transplantation of human iPSCs with mitotically inactivated feeder cells and Matrigel into immunodeficient mice.
 Fig.1 Schematic of teratoma formation. (Mohseni, R. 2014)
Fig.1 Schematic of teratoma formation. (Mohseni, R. 2014)
Teratoma Formation Assay
Studies have shown that the transplantation of iPSCs in an immunodeficient mouse will spontaneously differentiate to form a teratoma comprised of all three germ layers: endodermal, mesodermal, and ectodermal. As the most consistent property of iPSCs is the formation of teratomas, it has been served as the gold standard for evaluating pluripotency. The teratoma formation is strongly dependent on the sites of engraftment, including testis, liver, kidney capsule, hind leg muscle and the subcutaneous space. Based on the special needs of our customers, we can inject the iPSCs in different sites of immunodeficient mice. After the excision and fixation, the pluripotency can be determined by the presence of all three germ layers from the histology sections of the teratoma.
 Fig.2 Paraffin embedded tissue sections from a mouse iPSC teratoma are stained with hematoxylin and eosin. 3
Fig.2 Paraffin embedded tissue sections from a mouse iPSC teratoma are stained with hematoxylin and eosin. 3
Services at Creative Biolabs
Our teratoma formation assays for iPSCs are designed to characterize the pluripotency of iPSCs by assessing their ability to differentiate into cell types representative of the three germ layers: ectoderm, mesoderm, and endoderm. This service provides comprehensive analysis and validation for research purposes.
-
iPSC Preparation: Initial assessment of iPSC quality, including viability, morphology, and basic pluripotency marker expression (OCT4, SOX2, NANOG). Expansion and preparation of iPSC colonies for injection.
-
Animal Models: Selection of suitable immunodeficient mouse models (e.g., NOD/SCID, NSG) to minimize immune rejection of human cells. Proper acclimatization and care of animals.
-
Cell Injection: Injection of prepared iPSCs into immunodeficient mice, typically subcutaneously or intramuscularly. Monitoring of mice for teratoma development over a period of 4-12 weeks.
-
Teratoma Harvesting: Mice are euthanized humanely, and teratomas are carefully harvested. Macroscopic examination of teratomas for size, morphology, and location.
-
Histological Analysis: Fixation and paraffin embedding of harvested teratomas. Sectioning and staining of tissue samples (e.g., Hematoxylin and Eosin staining). Microscopic examination by experienced pathologists to identify tissue types from ectoderm, mesoderm, and endoderm.
-
Molecular Analysis: Immunohistochemistry (IHC) to detect specific differentiation markers. RNA sequencing or qPCR to assess gene expression profiles of differentiated tissues.
-
Reporting: Comprehensive report detailing all findings, including images of histological sections with annotations. Certification of iPSC pluripotency based on teratoma formation and tissue differentiation.
Our teratoma formation assays for iPSCs provide a reliable and comprehensive solution for validating the pluripotency of your iPSC lines, ensuring high-quality data for your research endeavors. Pricing is project-specific and depends on the scope of the study, number of samples, and additional analyses required. Please contact us for a detailed quote.
Protocol for Teratoma Formation Assay:
Features of Our Teratoma Formation Assay:
-
Short experimental cycle. (1-2 month)
-
Less number of cells required per injection site. (0.5 x 106 for mouse
-
The increased success rate of teratoma formation by kidney and testis injections.
-
Our team comprises experienced stem cell biologists and technicians specializing in iPSC characterization and teratoma formation.
-
We utilize advanced animal housing and surgical facilities to ensure high standards of care and precision in our procedures.
-
Detailed reports include histological analysis with high-resolution imaging, identification of tissue types from all three germ layers, and quantitative assessment of pluripotency markers.
-
Rigorous quality control procedures ensure reproducibility and reliability of results.
As a well-recognized expert in the field of iPSC generation and applications, Creative Biolabs is always dedicated to assisting our clients with the most satisfactory teratoma formation service. Moreover, we can also provide various services regarding iPSC technology, please do not hesitate to contact us for more details if you are interested in our services.
Published Data
Below are the findings presented in the article related to teratoma formation assays for iPSC.
Some researchers have explored whether CD133 is a potential target for reducing teratoma formation without radically altering differentiation. Hua Wang, et al. used CRISPR/Cas9 technology to construct the CD133 knockdown hESC WA26 cell line and tested CD133 knockdown and wild-type hESC cell lines for pluripotency, proliferation, telomere biology, and teratoma. They performed teratoma assays by hematoxylin and eosin (H&E) staining as well as immunohistochemistry and fluorescence microscopy of teratoma sections.
They found that CD133 is highly expressed in human ESC, but knockout of CD133 in hESC significantly attenuated hESC proliferation and teratoma formation without affecting hESC pluripotency or in vivo differentiation into three germ layers. CD133 can be used as an additional target and as a selective marker to sort and eliminate undifferentiated cells to reduce the potential risk of teratoma formation in hESC in regenerative medicine.
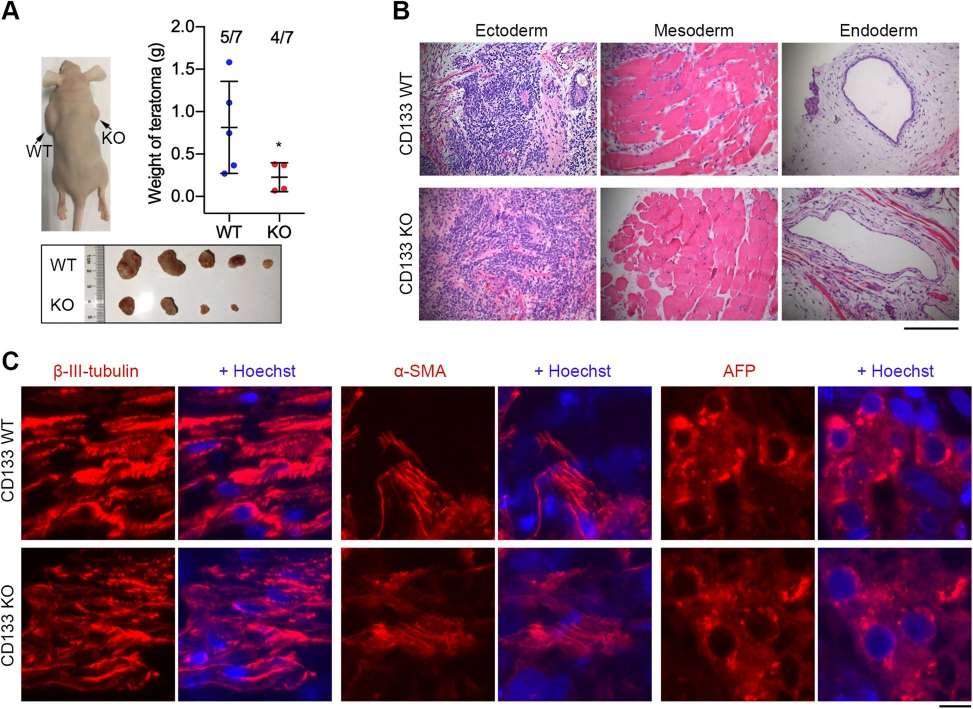 Fig. 3 Teratoma formation test following injection into nude mice.4
Fig. 3 Teratoma formation test following injection into nude mice.4
FAQs
-
Q: Are there any specific requirements for the iPSCs before they are submitted for the assay?
A: Yes, we require that the iPSCs are mycoplasma-free and have been tested for sterility. The cells should be well-characterized, including markers of pluripotency. We also recommend providing information about the culture conditions and any genetic modifications. This ensures that the cells are healthy and capable of forming teratomas, leading to reliable assay results.
-
Q: How many mice are typically used in a teratoma formation assay?
A: Typically, we use 3-5 immunodeficient mice per iPSC line for the teratoma formation assay. This number is chosen to ensure statistical significance and reproducibility of the results. Using multiple mice helps account for biological variability and ensures that the observed outcomes are representative of the iPSC line's pluripotency rather than anomalies or outliers.
-
Q: What are the costs associated with the teratoma formation assay, and what factors influence the pricing?
A: The cost of the teratoma formation assay depends on several factors, including the number of iPSC lines being tested, the specific requirements of the assay, and any additional services requested. We will provide a detailed quote after discussing your project in detail. Our pricing is competitive and reflects the comprehensive nature of our services, including animal care, histological analysis, and detailed reporting.
-
Q: How do you handle potential complications, such as the absence of teratoma formation?
A: If teratoma formation does not occur, we conduct a thorough investigation to determine the cause. This includes reviewing the quality and handling of the iPSCs, assessing the injection technique, and examining the health of the mice. We will provide a detailed report outlining our findings and recommendations. Depending on the cause, we may suggest repeating the assay or performing additional tests to resolve the issue.
Scientific Resources
References
-
Gropp, Michal, et al. "Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny." Plos One 7(9) (2012): e45532.
-
Hentze, Hannes, et al. "Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies." Stem cell research 2.3 (2009): 198-210.
-
Zhang, W.Y. "Teratoma formation: A tool for monitoring pluripotency in stem cell research." Current Protocols in Stem Cell Biology (2008) 4A.8.1-4A.8.17.
-
Wang, Hua, et al. "Role of CD133 in human embryonic stem cell proliferation and teratoma formation." Stem cell research & therapy 11 (2020): 1-14.
For Research Use Only. Not For Clinical Use.
 Fig.1 Schematic of teratoma formation. (Mohseni, R. 2014)
Fig.1 Schematic of teratoma formation. (Mohseni, R. 2014)
 Fig.2 Paraffin embedded tissue sections from a mouse iPSC teratoma are stained with hematoxylin and eosin. 3
Fig.2 Paraffin embedded tissue sections from a mouse iPSC teratoma are stained with hematoxylin and eosin. 3
 Fig. 3 Teratoma formation test following injection into nude mice.4
Fig. 3 Teratoma formation test following injection into nude mice.4