Cytokines are intercellular signal transduction molecules that closely mediate and adjust immune response, inflammatory reaction, promotion of hematopoietic cell differentiation and restoration of damaged tissues. Cytokines are involved in many different yet important pathways including the induction of T cell proliferation by IL-2, the induction of anti-viral protein expression by IFN gamma, and the inhibition of viral gene expression and replication by TNF alpha. The dysregulation of cytokine expression has been widely associated with many diseases and thus, the detection of cytokine expression levels is of vital importance for diagnosis as well as therapy development purposes.
The quantitative and activity detections of cytokine levels from a population of cells have been unable to provide enough information in some occasions. Therefore, a novel technology has been developed to meet these specific demands. Intracellular cytokine staining (ICS) assay coupled with flow cytometry can be utilized to detect the antigen-specific T cell responses at a single cell level for both clinical studies and scientific researches. This technology harnesses the particular advantage of enabling the simultaneous assessment of multiple phenotypic, differentiation and functional parameters pertaining to responding T cells, most notably, the expression of multiple effector cytokines.
Creative Biolabs has been providing ECIA™ intracellular cytokine staining services for customers all over the world for many years. With our extensive experience and expertise, your assays will be designed and conducted in the most efficient and cost-effective way. We realize that there are differences in various projects and objectives, therefore we frame our services in a highly-customized fashion by communicating with customers thoroughly for better solutions and optimization. We are willing to hear questions and suggestions from customers to make sure that the results of our services contribute to your project in a broader perspective.
ICS is achieved through blocking the secretion of protein mediated by Golgi apparatus by utilizing in vitro polyclonal activators or specific antigen-stimulated cells and cytokine transmembrane secretion blockers (e.g. brefeldin A), allowing the accumulation of cytokines in the cytoplasm. Fluorescence-labeled antibodies are then used to detect the cytokines. ICS can reflect characteristics of a single cell and display the specificity of cell groups, which directly indicates the in vivo cell state and the level of protein in the cytoplasm. After antibody staining, flow cytometry is used for analysis of the quantity and quality of relevant cells.
Other optional ECIA™ cellular analysis services:
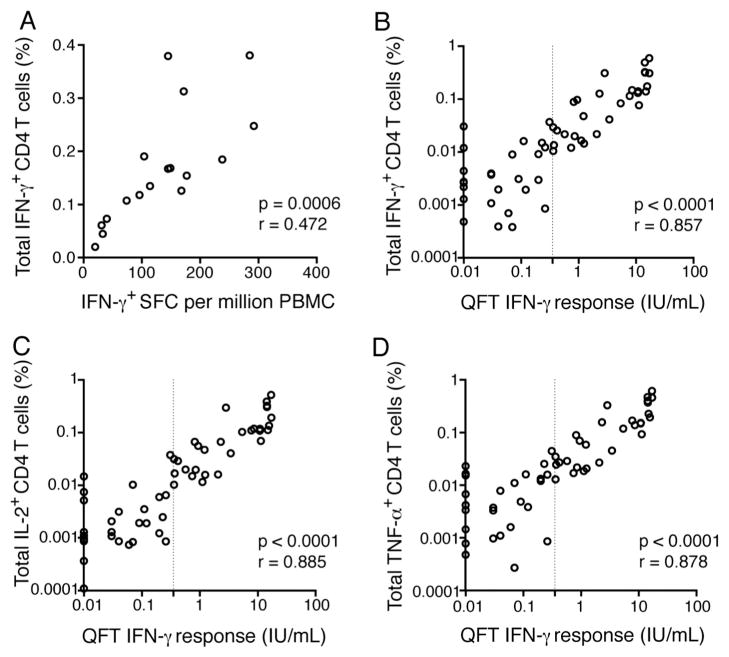 Fig. 1 Comparison of frequencies of Th1 cytokine-expressing CD4 T cells, measured by WB-ICS, with IFN-γ release detected by IFN-γ ELISpot assay or by QuantiFERON-TB Gold In-Tube assay (QFT). (Benjamin M. Kagina, 2015)
Fig. 1 Comparison of frequencies of Th1 cytokine-expressing CD4 T cells, measured by WB-ICS, with IFN-γ release detected by IFN-γ ELISpot assay or by QuantiFERON-TB Gold In-Tube assay (QFT). (Benjamin M. Kagina, 2015)
The article focuses on the qualification of a whole blood intracellular cytokine staining (ICS) assay for assessing mycobacteria-specific CD4 and CD8 T cell immunity, crucial for tuberculosis vaccine efficacy studies. The research demonstrates that the ICS assay is robust and reliable for quantifying specific T cell responses and shows that long-term cryopreservation of stimulated cells does not impair the assay's ability to detect cytokine expression. This finding is significant as it confirms the utility of the ICS technique in clinical trials and vaccine evaluations, especially in settings where batch testing is necessary, thus ensuring that variations in sample handling do not affect the assessment of vaccine immunogenicity.
Intracellular cytokine staining is a flow cytometry technique used to detect and quantify cytokine production at the single-cell level within immune cells. This method allows researchers to assess the functional status of cells by measuring their cytokine response after antigen stimulation, providing insights into cellular immune responses.
ICS involves stimulating immune cells with antigens or mitogens to induce cytokine production. The cells are then treated with a protein transport inhibitor like Brefeldin A, which prevents cytokine secretion, trapping them inside the cells. Following fixation and permeabilization, specific antibodies labeled with fluorescent markers are used to stain the cytokines inside the cells.
ICS is widely used in immunology research to assess the functionality of T cells, B cells, and other cytokine-producing cells in various settings, including vaccine development, infectious disease research, and autoimmune disorders. It helps determine the efficacy of vaccines and therapies by analyzing how immune cells respond to pathogens or treatments.
ICS provides a distinct advantage by allowing the detection of cytokines at the single-cell level, offering a detailed profile of cytokine expression within different cell subsets. Unlike ELISA or cytokine bead arrays, ICS can simultaneously measure multiple cytokines in individual cells, revealing the polyfunctionality of cells which is crucial for understanding complex immune responses.
The primary challenges include the need for proper cell stimulation, the use of specific antibodies, and the necessity for precise cell permeabilization and fixation techniques to preserve cell morphology and cytokine localization. Additionally, extensive optimization is often required to reduce background staining and improve the assay's specificity and sensitivity.
Selecting high-quality, specific antibodies that are conjugated with appropriate fluorochromes is crucial for successful ICS. Antibodies must be validated for intracellular staining to ensure they can recognize cytokines within the cell after the permeabilization process. The choice of fluorochrome also impacts the assay sensitivity due to varying levels of brightness and photostability.
Data from an ICS assay are interpreted by analyzing the fluorescence intensity of stained cells using flow cytometry. This analysis provides information about the percentage of cells producing specific cytokines and the amount of cytokine each cell produces, which can be correlated with cellular responses to infection, vaccination, or therapeutic intervention.
ICS can detect a wide range of cytokines, including but not limited to IFN-gamma, IL-2, IL-4, IL-10, IL-17, and TNF-alpha. The flexibility to detect multiple cytokines simultaneously allows researchers to assess various aspects of cellular immune responses and identify different T cell subsets based on their cytokine profiles.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
