Acinetobacter baumannii-derived Exosome Research and Application
Research on Acinetobacter baumannii-derived exosomes inducing protective immune responses is beneficial to enhance their application in vaccine development. Creative Biolabs possesses specialized exosome research teams that can advance projects related to bacterial-derived exosomes.
Isolation of Acinetobacter baumannii-derived Exosomes
-
Acinetobacter baumannii was cultured in Mueller Hinton medium at 37°C with shaking overnight, whereas for the culture of lpxD mutant strains of Acinetobacter baumannii lacking LPS synthesis, the addition of colistin to the culture medium as a selective pressure in favor of lpxD mutation maintenance.
-
Centrifuge the Acinetobacter baumannii broth at high speed, then collect and filter the supernatant.
-
After centrifuging the Acinetobacter baumannii supernatant again at ultra-high speed, dissolve the Acinetobacter baumannii-derived exosomes precipitate in PBS.
-
Filter Acinetobacter baumannii-derived exosomes to sterilize, verify product sterility by agar plate culture, and verify lower endotoxin levels with horseshoe crab reagents.
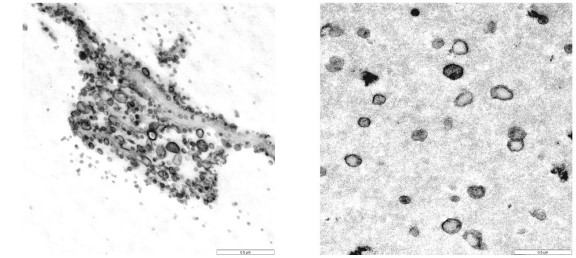 Fig. 1 TEM of exosomes derived from Acinetobacter baumannii and from mutant strains of Acinetobacter baumannii lacking LPS synthesis.1
Fig. 1 TEM of exosomes derived from Acinetobacter baumannii and from mutant strains of Acinetobacter baumannii lacking LPS synthesis.1
Studies on Acinetobacter baumannii-derived Exosomes
|
Research
|
Conclusion
|
|
Acinetobacter baumannii-derived exosomes induced antibody production after immunization.
|
Intramuscular injection of Acinetobacter baumannii-derived exosomes induced a significant increase in the level of antigen-specific antibodies in immunized mice, and the total antibody potency and antibody titer increased with booster immunization.
|
|
Acinetobacter baumannii-derived exosomes contributed to the reduction of bacterial load and inhibition of inflammation in mice.
|
Mice immunized with Acinetobacter baumannii-derived exosomes had significantly lower bacterial loads and higher survival rates compared to control mice after infection with specific antigens. ELISA of serum cytokine levels revealed a significant reduction in proinflammatory cytokine release in mice vaccinated with Acinetobacter baumannii-derived exosomes.
|
|
Intramuscular immunization with exosomes derived from clinical isolates partially protected the mice from infection by specific antigens.
|
Several clinical isolates related to Acinetobacter baumannii were isolated from patients' bronchoalveolar exchange fluid and the respiratory attack of the clinical isolates was modeled. Quantification of antibodies and inflammatory factors showed that exosomes derived from these clinical isolates could only partially protect infected mice by immunization with intramuscular injections.
|
|
Subcutaneous injection elicited a greater immunological response than intramuscular injection.
|
By comparing the immunization modalities of subcutaneous and intramuscular injections on bacterial load and proinflammatory factor levels in infected mice, subcutaneous injections showed a superior protective effect to intramuscular injections.
|
|
Nasal immunization enhanced nasal resistance to infection in mice.
|
Nasal immunization of mice with Acinetobacter baumannii-derived exosomes was shown to generate specific mucosal immune responses and induce local IgA responses. Moreover, compared with other immunization modalities, nasal immunization provided the strongest protection to mice with nasal infections, completely preventing the systemic spread of bacteria from the lungs to the spleen.
|
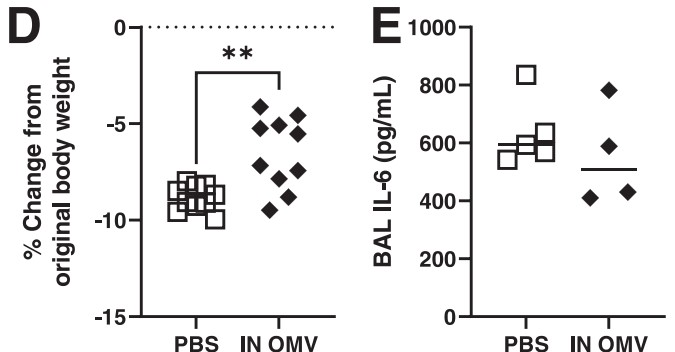 Fig. 2 Nasal administration of Acinetobacter baumannii-derived exosomes reduced body weight loss and pro-inflammatory factor levels in mice.2
Fig. 2 Nasal administration of Acinetobacter baumannii-derived exosomes reduced body weight loss and pro-inflammatory factor levels in mice.2
Acinetobacter baumannii-derived exosomes have been studied to trigger protective immune responses and contrast efficacy in different modes of administration, facilitating the clinical translation of the bacterial vesicles. Creative Biolabs is committed to providing clients with professional services for additional Acinetobacter baumannii-derived exosome research. Please contact us with interest.
References
-
Pulido, Marina R., et al. "A lipopolysaccharide-free outer membrane vesicle vaccine protects against Acinetobacter baumannii infection." Vaccine 38.4 (2020): 719-724.
-
Higham, Sophie L., et al. "Intranasal immunization with outer membrane vesicles (OMV) protects against airway colonization and systemic infection with Acinetobacter baumannii." Journal of Infection 86.6 (2023): 563-573.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 TEM of exosomes derived from Acinetobacter baumannii and from mutant strains of Acinetobacter baumannii lacking LPS synthesis.1
Fig. 1 TEM of exosomes derived from Acinetobacter baumannii and from mutant strains of Acinetobacter baumannii lacking LPS synthesis.1
 Fig. 2 Nasal administration of Acinetobacter baumannii-derived exosomes reduced body weight loss and pro-inflammatory factor levels in mice.2
Fig. 2 Nasal administration of Acinetobacter baumannii-derived exosomes reduced body weight loss and pro-inflammatory factor levels in mice.2








