Escherichia coli-derived Exosome Research and Application
Escherichia coli-derived exosomes have been found to mediate crosstalk between their parent bacterium and the host to induce disease by manipulating the host's autophagic and inflammatory response. Creative Biolabs specializes in the study of bacterial-derived exosomes and can provide high-quality research services to assist in the understanding of bacterial vesicle-mediated pathogenesis.
Escherichia coli-derived Exosome Isolation
-
Incubate Escherichia coli suspension for 8-10 hours.
-
Centrifuge the Escherichia coli culture at 4°C at low speed.
-
Filter the Escherichia coli supernatant to remove residual bacteria.
-
Ultrafiltrate and concentrate Escherichia coli supernatant.
-
Ultracentrifuge the concentrated supernatant to obtain Escherichia coli-derived exosomes.
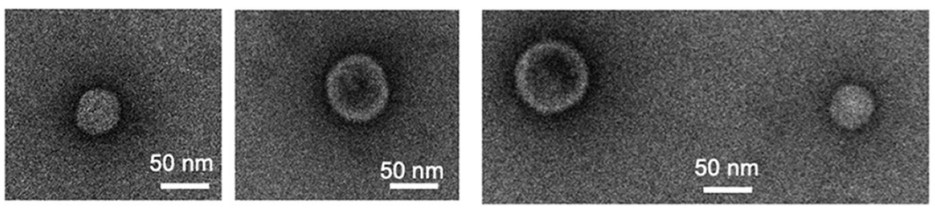 Fig. 1 Transmission electron microscopy of Escherichia coli-derived exosomes.1
Fig. 1 Transmission electron microscopy of Escherichia coli-derived exosomes.1
Research on Escherichia coli-derived Exosome
|
Research
|
Conclusion
|
|
Exosomes isolated from Escherichia coli expressing the virulence factor HlyF promote cellular LC3-positive increases in a dose-dependent manner.
|
Comparison of intracellular LC3 foci treated with exosomes from Escherichia coli expressing HlyF and HlyF mutated revealed that the level of LC3-positive structures and LC3-II were enhanced in dependence on exosome parent bacterium HlyF expression.
Moreover, GFP-LC3 fluorescence quantification showed that HlyF-expressing Escherichia coli-derived exosomes caused the accumulation of LC3-positive structures and increased intracellular autophagosomes in a dose-dependent manner. In contrast, HlyF-mutated Escherichia coli-derived exosomes did not cause an increase in LC3 levels.
|
|
HlyF-positive Escherichia coli-derived exosomes triggered high levels of LC3 by specific autophagosome accumulation.
|
Co-localization observations, transmission electron microscopy observations, and phagocytosis inhibitor treatment revealed that LC3-positive vesicles induced by HlyF-positive Escherichia coli-derived exosomes presented double membranes indicative of autophagosomes rather than single membrane phagocytosis vesicles, and that the increase in LC3-II was unaffected by phagocytosis inhibitors. This indicated that HlyF-positive Escherichia coli-derived exosomes triggered the accumulation of autophagosomes in cells.
|
|
HlyF-positive Escherichia coli-derived exosomes induced autophagosome accumulation resulting from autophagy blockade.
|
Quantification showed that blockade of autophagosome degradation with chloroquine did not cause a further increase in LC3, suggesting that the accumulation of autophagosomes induced by HlyF-positive Escherichia coli-derived exosomes results from autophagy blockade rather than autophagic flux. Further experiments based on reporter cells expressing acid-sensitive and acid-stabilized fluorescent proteins revealed that this autophagy blockage mainly refers to the suppression of autophagy-lysosome fusion and autophagosome clearance.
|
|
HlyF-positive Escherichia coli-derived exosomes caused blockade of cellular autophagy and subsequently exacerbated activation of noncanonical inflammasomes.
|
Cell death and release of IL1B from bone marrow-derived macrophages of Escherichia coli-derived exosomes-treated mice was detected, which was counteracted in inflammasome gene mutant mice. It suggests that such autophagy blockade is followed by activation of the noncanonical inflammasome pathway.
|
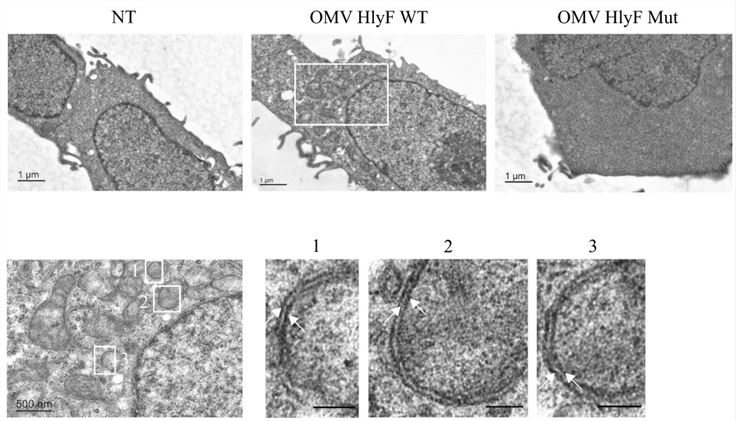 Fig. 2 Transmission electron microscopy observation of double-membrane autophagosomes in HlyF-positive Escherichia coli-derived exosomes-treated cells.2
Fig. 2 Transmission electron microscopy observation of double-membrane autophagosomes in HlyF-positive Escherichia coli-derived exosomes-treated cells.2
The mechanisms of Escherichia coli-derived exosomes delivering virulence involve blocking autophagic fluxes on the one hand, and promoting inflammation on the other hand. Creative Biolabs provides reliable vesicle research solutions to advance Escherichia coli-derived exosome research and applications. Please contact us to get a quote.
References
-
Imamiya, Risa, et al. "Escherichia coli-derived outer membrane vesicles relay inflammatory responses to macrophage-derived exosomes." Mbio 14.1 (2023): e03051-22.
-
David, Laure, et al. "Outer membrane vesicles produced by pathogenic strains of Escherichia coli block autophagic flux and exacerbate inflammasome activation." Autophagy 18.12 (2022): 2913-2925.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Transmission electron microscopy of Escherichia coli-derived exosomes.1
Fig. 1 Transmission electron microscopy of Escherichia coli-derived exosomes.1
 Fig. 2 Transmission electron microscopy observation of double-membrane autophagosomes in HlyF-positive Escherichia coli-derived exosomes-treated cells.2
Fig. 2 Transmission electron microscopy observation of double-membrane autophagosomes in HlyF-positive Escherichia coli-derived exosomes-treated cells.2








