Immune Antibody Libraries in Phage Display
Introduction Comparison Construction & Screening Applications
Introduction to Immune Antibody Libraries
What are Immune Antibody Libraries?
Immune antibody libraries contain unique sets of antibody genes which originate from B cells taken from donors who have been immunized or who have experienced natural infection. Antibody libraries provide pre-optimized antibodies with high affinity and specificity against specific antigens because of previous immune exposure. The development of therapeutic antibodies from immune libraries eliminates the necessity for in vivo animal immunization.
Mechanism of Antibody and Coat Protein Fusion Display
The phage display method enables antibody fragments to be displayed on bacteriophage surfaces by genetically linking them to coat proteins such as pIII or pVIII found in M13 filamentous phages. The system facilitates fast identification and selection of antigen-specific antibodies by conducting multiple rounds of biopanning.
The fusion mechanism works as follows:
-
Insertion of antibody genes into a phagemid vector containing the gene for a phage coat protein.
-
Expression of the fusion protein—antibody fragments displayed on the phage surface.
-
Binding to the target antigen followed by washing off non-specific binders.
-
Elution of high-affinity binders, leading to their enrichment through multiple selection rounds.
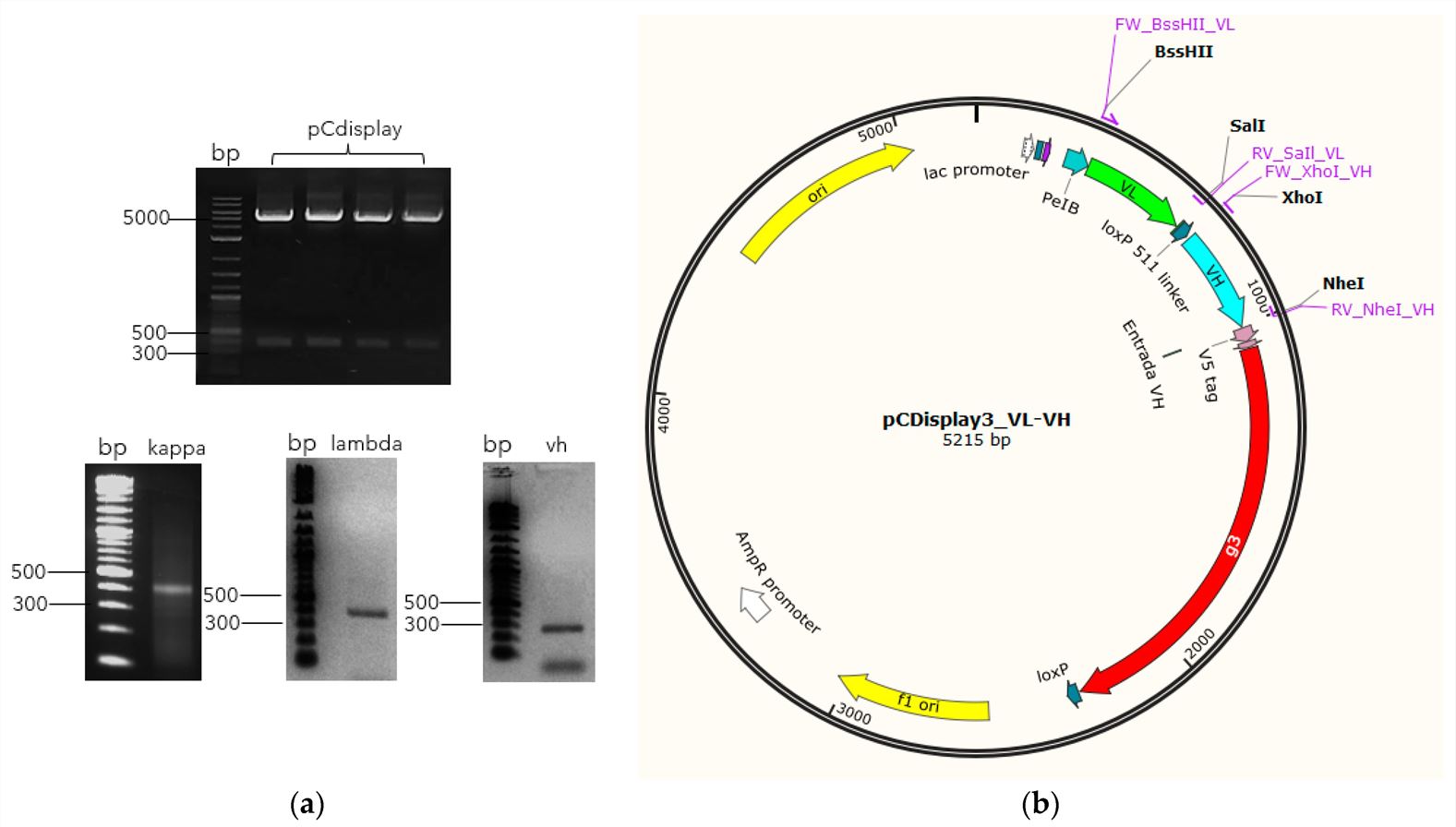 Fig. 1 Library assembly schematic.1, 3
Fig. 1 Library assembly schematic.1, 3
Sources of Immune Library Construction
Immune libraries are typically constructed from B cell-derived mRNA isolated from immune donors. The two major approaches are:
-
Natural Immune Library: Derived from patients recovering from infections or vaccinated individuals.
-
Artificially Boosted Immune Library: Generated from experimental animal models or subjects intentionally exposed to specific antigens.
Key steps include:
-
mRNA extraction from peripheral blood B cells or spleen.
-
Reverse transcription to cDNA and amplification of variable heavy (VH) and variable light (VL) chain sequences.
-
Cloning into phagemid vectors for expression in E. coli host cells.
Types of Immune Libraries
Immune libraries are classified based on the source of antibody genes:
|
Library Type
|
Description
|
Applications
|
|
Human Immune Library
|
Derived from human donors post-infection or vaccination
|
Therapeutic antibody discovery, clinical diagnostics
|
|
Humanized Library
|
Genetically modified to replace animal-derived framework regions with human sequences
|
Reduced immunogenicity in human therapy
|
|
Animal-Derived Library
|
Constructed from immunized animals such as mice, rabbits, or camels
|
Used in preclinical research, cross-species antibody generation
|
Comparison of Immune Libraries with Other Library Types
Advantages and Limitations of Immune vs. Naïve vs. Synthetic Libraries
|
Feature
|
Immune Library
|
Naïve Library
|
Synthetic Library
|
|
Affinity
|
High, due to immune selection
|
Low, requires in vitro maturation
|
Variable, depends on design
|
|
Diversity
|
Moderate, limited to donor response
|
Extremely high
|
Tailored, but potentially biased
|
|
Antigen Specificity
|
High, optimized by immune exposure
|
Broad but low-affinity
|
Designed for selected targets
|
|
Immunogenicity Risks
|
Low
|
Low to moderate
|
Variable, depends on framework
|
Unique Value of Immune Library in Targeting Specific Antigens
-
Cancer Antigens: Immune libraries derived from cancer patients may contain highly specific antibodies against tumor neoantigens.
-
Self-Antigens: Autoimmune disease-related libraries can yield antibodies targeting disease-relevant self-proteins, which may be challenging to obtain via immunization.
Technological Advantages of Immune Antibody Libraries
-
No Need for Animal Immunization: Immune libraries harness human immune responses which eliminates both ethical and technical limitations of animal immunization methods.
-
Handling Toxic/Non-Immunogenic Antigens: Immune phage display provides a method to select antibodies against toxic proteins such as bacterial toxins and venom components as well as weakly immunogenic antigens like carbohydrates and lipids and self-antigens without triggering animal tolerance mechanisms unlike traditional immunization techniques.
-
Shortened Screening Time: The phage display technique fast-tracks antibody discovery by identifying lead candidates within 6–8 weeks unlike the hybridoma-based methods which take several months.
Immune Library Construction and Screening Process
Immune Library Construction Steps
-
Donor B Cell Isolation: Convalescent patients, vaccinated individuals or immunized animals serve as immune donors. B cells (CD19+ or CD138+) are isolated from PBMCs via density gradient centrifugation and MACS/FACS sorting.
-
mRNA Extraction & cDNA Synthesis: Total RNA is extracted using TRIzol or silica-based methods. Reverse transcription generates cDNA, encoding the antibody genes.
-
Antibody Gene Amplification & Assembly: VH and VL genes are amplified by PCR using framework-specific primers. Antibody fragments are assembled as scFv, Fab, or sdAb (single domain antibody).
-
Cloning into Phagemid Vectors: Antibody genes are inserted into phagemid vectors (e.g., pComb3X, pHEN) containing pIII or pVIII coat protein fusion sequences or M13 origin of replication & antibiotic resistance gene.
-
Transformation & Phage Rescue: E. coli cells (TG1, SS320, XL1-Blue) are transformed with the phagemid. Helper phage (M13KO7) infection induces phage particle assembly. The phage-displayed library is harvested at 1012 pfu/mL.
Biopanning: Screening for High-Affinity Antibodies
-
Target Antigen Immobilization: Antigen-coated surfaces (ELISA plates, beads, or cell-based display). Blocking with BSA or casein to reduce non-specific interactions.
-
Phage Binding & Washing: Incubation with antigen (30 min–2 hr). Sequential washing to remove weak binders.
-
Elution of High-Affinity Binders: Acidic elution (Glycine-HCl, pH 2.2) or competitive elution (free antigen at excess concentration).
-
Amplification & Enrichment: Eluted phages infect E. coli for propagation. Repeated for 3–5 rounds to enrich high-affinity clones.
-
Screening & Characterization: Phage ELISA identifies specific antigen binders, sequencing ensures diversity, and SPR/BLI (Surface Plasmon Resonance, Biolayer Interferometry) confirms affinity.
Table 1. Summary of Immune Library Construction and Screening Workflow.
|
Step
|
Key Actions
|
Outcome
|
|
1. Donor B Cell Collection
|
Isolate B cells from immune donors
|
Immune B cell population
|
|
2. RNA Extraction & cDNA Synthesis
|
Extract mRNA, reverse transcribe to cDNA
|
Antibody gene template
|
|
3. PCR Amplification & Assembly
|
Amplify VH/VL, assemble scFv, Fab, or sdAb
|
Functional antibody fragments
|
|
4. Cloning into Phagemid Vectors
|
Insert genes into display vectors
|
Phagemid library
|
|
5. Transformation & Phage Rescue
|
Express in E. coli, infect with helper phage
|
Displayed library (~1012 diversity)
|
|
6. Biopanning
|
Binding, washing, elution of phages
|
Enrichment of specific antibodies
|
|
7. Characterization
|
ELISA, sequencing, affinity measurements
|
Lead antibody candidates
|
Applications of Immune Library Phage Display
Immune library phage display technology has become a powerful tool in biotechnology, offering high specificity and rapid antibody selection for various applications. This approach harnesses the natural immune response of donors, providing antibodies with pre-optimized affinity and specificity against target antigens.
 Fig. 2 Monoclonal antibody clone characterization.2, 3
Fig. 2 Monoclonal antibody clone characterization.2, 3
Therapeutic Antibody Discovery and Development
Therapeutic antibodies represent one of the most successful biologic drug classes, with applications spanning oncology, infectious diseases, and autoimmune disorders. Immune phage display accelerates the development of fully human, high-affinity antibodies.
-
High specificity and affinity: Antibodies are already optimized by the immune system.
-
Human-derived libraries: Avoid immunogenicity issues associated with animal-derived antibodies.
-
Rapid lead generation: Selection time reduced to 6-8 weeks compared to traditional hybridoma technology.
Diagnostic Applications in Disease Detection
The high specificity of immune-derived antibodies makes them invaluable for early disease detection and diagnostics.
-
Rapid antibody identification for new infectious diseases.
-
High specificity to distinguish closely related antigens (e.g., different viral strains).
-
Long-term stability in diagnostic kits.
Research Tools for Studying Protein Interactions
Phage-displayed immune libraries serve as a powerful tool for protein interaction studies, aiding in:
-
Epitope mapping – Identifying antigenic regions for vaccine design.
-
Protein-protein interaction studies – Understanding receptor-ligand binding.
-
Structure-function analysis – Determining key amino acid residues in immune recognition.
Emerging Applications: Personalized Medicine & Synthetic Biology
Beyond traditional uses, immune phage display is now being integrated into next-generation applications such as:
Patient-derived antibody libraries for custom cancer therapies.
Predicting treatment responses using patient-specific antibody profiles.
-
Synthetic Biology & Engineered Antibodies
Chimeric antigen receptor (CAR) discovery for CAR-T and CAR-NK therapies.
De novo design of synthetic immune receptors for engineered cell therapies.
Table 2. Summary of Immune Phage Display Library Applications
|
Application
|
Key Uses
|
Examples
|
|
Therapeutic Antibody Discovery
|
Monoclonal antibodies for cancer, infectious diseases, autoimmune disorders
|
PD-1 inhibitors, SARS-CoV-2 neutralizers, TNF-α blockers
|
|
Diagnostics
|
Disease detection and biomarker identification
|
COVID-19 rapid tests, early cancer detection kits
|
|
Research Tools
|
Protein interaction studies, epitope mapping
|
Structural biology, proteomics
|
|
Emerging Fields
|
Personalized medicine, synthetic biology
|
CAR-T therapy, custom antibody design
|
Immune library phage display technology has revolutionized antibody discovery and development, with applications in therapeutics, diagnostics, and research. The ability to rapidly isolate high-affinity antibodies without the need for animal immunization has positioned this technology as a cornerstone of biopharmaceutical innovation. Creative Biolabs leads in cutting-edge immune phage display library services. Contact us to leverage our expertise in custom antibody discovery solutions!
Learn more about Creative Biolabs phage display services:
References
-
Effer, Brian, et al. "Construction of a human immune library from gallbladder cancer patients for the single-chain Fragment Variable (scFv) antibody selection against claudin 18.2 via phage display." Antibodies 13.1 (2024): 20. https://doi.org/10.3390/antib13010020
-
Rahumatullah, Anizah, et al. "Broad specificity of immune helminth scFv library to identify monoclonal antibodies targeting Strongyloides." Scientific Reports 11.1 (2021): 2502. https://doi.org/10.1038/s41598-021-82125-3
-
Distributed under Open Access license CC BY 4.0, without modification.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
 Fig. 1 Library assembly schematic.1, 3
Fig. 1 Library assembly schematic.1, 3
 Fig. 2 Monoclonal antibody clone characterization.2, 3
Fig. 2 Monoclonal antibody clone characterization.2, 3
