The molecular approach of phage display enables peptides, proteins, and antibody fragments to be displayed on bacteriophage surfaces. Genetically modified bacteriophages function to connect displayed antibody phenotypes with their encoding DNA genotypes which allows for effective high-affinity binder selection against target antigens.
| Year | Milestone |
| 1985 | George P. Smith develops the first phage display method using filamentous phage. |
| 1990 | Use of phage display for antibody engineering begins. |
| 2002 | First FDA-approved fully human antibody derived via phage display. |
| 2018 | Nobel Prize in Chemistry awarded to Smith and Winter for phage display contributions. |
Phage display relies on the insertion of DNA encoding antibody fragments (e.g., scFv or Fab) into the phage genome, resulting in the surface display of these fragments. Phages with high-binding specificity to a target are enriched via biopanning, a process involving:
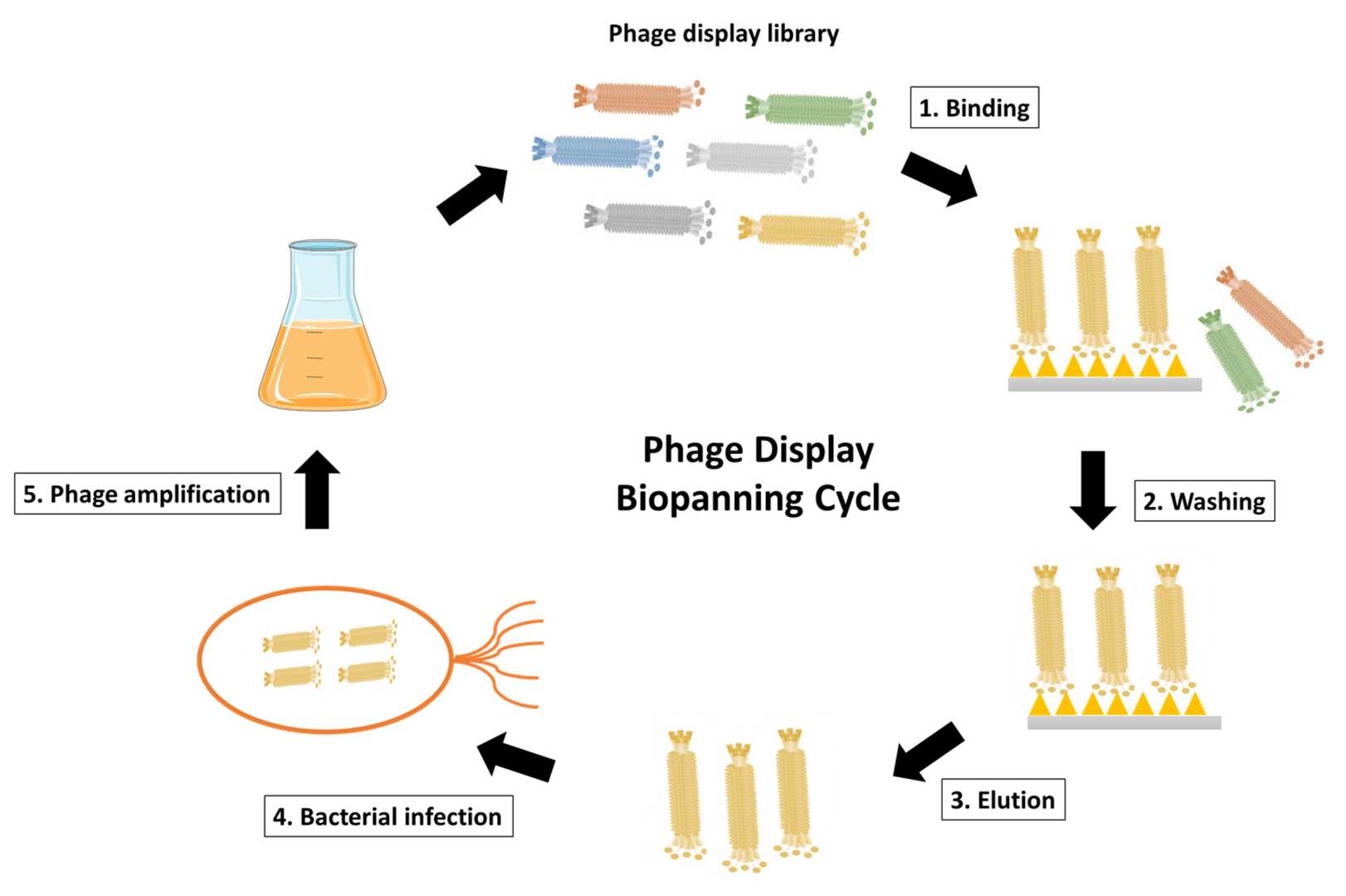 Fig. 1 Schematic representation of phage display biopanning cycle.1, 3
Fig. 1 Schematic representation of phage display biopanning cycle.1, 3
Creating strong and varied phage display libraries plays an essential role in high-throughput antibody discovery and engineering during human monoclonal antibody development. Through these libraries scientists can select high specificity and affinity antibodies against any target without needing animal immunization. Different types of human antibody phage display libraries emerge from their molecular format and antibody diversity sources.
Human scFv antibody libraries contain immunoglobulin variable regions from heavy (VH) and light (VL) chains joined together by a short peptide linker that usually adopts a (G₄S)₃ configuration. The compact structure maintains antigen-binding capability together with efficient expression and simplified genetic manipulation.
Key Advantages:
Human Fab antibody libraries include both the variable regions and the first constant domains (CH1 and CL) of immunoglobulin heavy and light chains. This format more closely resembles the native conformation of antibodies, offering improved stability and functional fidelity.
Key Advantages:
Human phage display libraries can also be categorized based on the origin of the antibody genes—whether they are derived from naive (non-immunized) or immune (antigen-experienced) B cells—or synthetically designed.
Derived from healthy, non-immunized donors, naive libraries capture the broad, natural diversity of the human immune repertoire.
| Feature | Naive Libraries |
| Source | Peripheral blood or bone marrow from healthy donors |
| Diversity | Very high (10⁹–10¹¹ unique clones) |
| Antigen Specificity | Random, not pre-enriched |
| Application | Universal screening for novel targets |
| Advantage | Ideal for first-in-class antibody discovery without prior antigen exposure |
Constructed from B cells of donors who have been exposed to a particular antigen, these libraries are enriched for high-affinity, antigen-specific antibodies.
| Feature | Immune Libraries |
| Source | B cells from immunized individuals or convalescent patients |
| Diversity | Moderate, with focused specificity |
| Antigen Specificity | Highly enriched |
| Application | Discovery of mature antibodies against known pathogens or diseases |
| Advantage | High hit rate for target-specific antibodies |
Synthetic and semi-synthetic libraries are engineered entirely in vitro, using optimized human germline frameworks and artificially diversified complementarity-determining regions (CDRs).
| Library Type | Description | Key Benefits |
| Synthetic | Fully artificial CDR design and assembly | Fully customizable; avoids immune tolerance |
| Semi-synthetic | Natural frameworks with synthetic CDRs | Combines diversity with natural folding |
| Engineered | Tailored for hard-to-target antigens | Enables focused selection and rational design |
Step-by-Step Workflow:
A clear comprehension of the structural and functional differences between scFv and Fab libraries helps researchers choose the proper format for their specific application.
| Feature | scFv Library | Fab Library |
| Structure | VH-linker-VL (single chain) | VH-CH1 + VL-CL (two chains) |
| Molecular Size | ~25–30 kDa | ~50–55 kDa |
| Folding | Less native; prone to aggregation | More native-like folding |
| Stability | Moderate | Higher biochemical stability |
| Expression | High yield in E. coli | Moderate; sometimes lower |
| Applications | CARs, diagnostic reagents, bispecifics | Therapeutic antibody leads, structural studies |
Successful phage display libraries depend fundamentally on the presence of diversity. An extensive variety in antibody repertoires boosts the probability of finding high-affinity binders that attach to a wide range of antigens and even challenging non-immunogenic targets.
Table 1. Key Diversity Generation Techniques
| Technique | Purpose | Notes |
| Donor Pooling | Broaden germline coverage | Combining B cells from 50–200 donors boosts VH/VL diversity |
| Chain Shuffling | Reassort natural VH and VL combinations | Allows recombination of heavy/light chains from different clones |
| CDR Randomization | Introduce synthetic variability in CDR loops | Used in synthetic/semi-synthetic libraries |
| Error-Prone PCR | Mimics somatic hypermutation | Enables in vitro affinity maturation |
| Framework Engineering | Use stable human scaffolds | Improves expression, folding, and downstream development |
| In Silico Design | Predict structure-function outcomes | Machine learning models help design focused libraries |
Human antibody phage display libraries have transformed therapeutic antibody research by providing an animal-free and scalable system to produce fully human monoclonal antibodies with high controllability. Today's blockbuster biologic medicines rely on these libraries which enable precise therapeutic targeting throughout numerous disease categories.
Phage display allows in vitro selection of human antibodies against any target of interest, including:
 Fig. 2 Generation and specificity of anti-SIRPα mAbs.2, 3
Fig. 2 Generation and specificity of anti-SIRPα mAbs.2, 3
Why phage display is ideal for therapeutic design:
| Feature | Benefit |
| Fully human antibodies | Minimizes immunogenicity risk |
| High-throughput selection | Accelerates lead discovery |
| In vitro screening | Independent of immune response limitations |
| Synthetic diversity | Access to rare or non-natural binding profiles |
| Modular design | Facilitates bispecific or antibody-drug conjugate (ADC) engineering |
The typical therapeutic antibody development process, when powered by phage display, involves a structured pipeline:
Human antibody libraries continue to play a critical role in the clinical pipeline, with phage-derived antibodies advancing across a wide range of therapeutic areas:
Phage display continues to be essential for developing human monoclonal antibodies intended for therapeutic and industrial uses. Phage display technology will lead the charge in producing next-generation biologics because advancements in library design, affinity maturation, and platform integration provide superior precision and scalable production capabilities. Creative Biolabs leads antibody discovery by providing tailored phage display solutions along with library construction and therapeutic antibody development for clients worldwide.
Learn more about Creative Biolabs custom phage display services and premade phage display library licensing services:
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
