T cells recognize linear amino acid sequences (or epitopes) derived from protein processed and presented in the context of self-major histocompatibility complex (MHC) protein. The aim of T cell epitope mapping is to identify the shortest amino acid sequence of a specific antigen recognized by CD4+ helper T cells or CD8+ cytotoxic T cells. In human beings, MHC class I molecules residing on most nucleated somatic cells present peptides to CD8+ T cells, while MHC class II molecules sitting on the professional antigen-presenting cells especially present peptides to CD4+ T cells.
Epitopes presented by MHC molecules are slightly different in length. MHC class I molecules typically present peptides for 8 to 11 amino acids, whereas MHC class II molecules present longer ones for 13-17 amino acids. T cell epitopes presented on MHC I or MHC II molecules potentially triggers a long lasting as well as exclusive cytotoxic immune response, which is essential for immune responses of infections, diseases, cancers and other pathogens. Based on this fact, epitope mapping emerges as a robust tool for vaccine design, drug development or disease treatment (e.g. cancer treatment, autoimmune disease) by which researchers could figure out the crucial peptide fragments of some diseases and study for the further purpose.
 Fig 1. T-cell epitope presented by MHC II.
Fig 1. T-cell epitope presented by MHC II.
Combined with the advantages of various T cell epitope discovery strategies, Creative Biolabs' excellent researchers have developed the CreMap™ MHC-peptide binding assay service for T cell epitope discovery based on the multimers screening and ELISpot assay, which could meet our customers' various demands. It’s a novel technology for rapidly detecting potential T cell epitopes of proteins and validating those epitopes in weeks.
In addition, we have generated a novel CreMap™ Antigen Processing Assay to measure the antigen processing. This assay is based on analyzing the degradation activity or proteolytic activity of screened peptide or proteins. The studies conducted by our labs have demonstrated that our assay is critical to identifying the T cell epitope, understanding the potential immunogenicity of target candidates in drug development.
Our CreMap™ T cell epitope discovery platform provides highly sensitive and reproducible assay service, including high throughput assays of MHC class I or MHC class II binding peptides, antigen-specific CD4+ and CD8+ T cell immune responses assays. For personalized service, our research team will customize an optimal proposal for our clients to achieve the best experimental results and simultaneously save your money and time.
Based on our advanced technical platform, Creative Biolabs is confident in delivering a quick and cost-effective T cell epitope mapping analysis service for our clients. If you are interested in the details of our CreMap™ T Cell Epitope Discovery Service, please contact us for more information.
Learn more about CreMap™ T cell epitope discovery services:
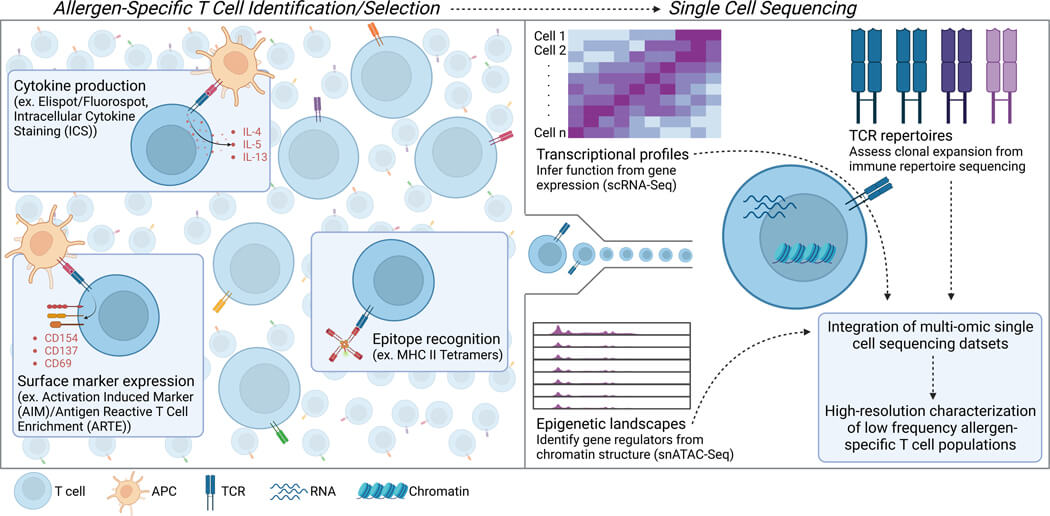 Fig. 2 Schematic representation of allergen-specific T cell identification/selection and downstream single cell sequencing applications.1,2
Fig. 2 Schematic representation of allergen-specific T cell identification/selection and downstream single cell sequencing applications.1,2
The article explores the significant role of T cells in food allergies, emphasizing a lack of detailed understanding of the T cells contributing to allergic reactions. The research underscores the importance of T cell epitope discovery enabled by advancements in single cell technologies. This approach aids in identifying and characterizing rare antigen-specific T cells at a single-cell level, which is crucial for diagnosing and potentially treating food allergies more effectively. The identification of T cell epitopes is especially pivotal as it allows for a clearer mechanistic understanding of T cell responses to food allergens, potentially leading to targeted therapies that can modify these responses and improve clinical outcomes in food allergy treatments.
A T-cell epitope is a short sequence of amino acids on an antigen that is recognized by T-cell receptors (TCRs). These epitopes are crucial in triggering an immune response, as T-cells play a central role in identifying and combating pathogens and other foreign molecules.
T-cell epitope discovery helps identify the specific parts of an antigen that elicit a strong immune response. By incorporating these epitopes into vaccines, researchers can create more effective vaccines that can specifically target pathogens and enhance the immune system's ability to fight disease.
Advanced technologies such as peptide-MHC multimer staining, T-cell cloning, and high-throughput sequencing of T-cell receptors are employed in T-cell epitope discovery. These methods enable the detailed analysis of T-cell responses and the identification of epitopes that are critical for developing targeted immunotherapies and vaccines.
T-cell epitope mapping can reveal how certain autoimmune diseases occur when T-cells mistakenly target the body's own tissues. Identifying these autoreactive T-cell epitopes helps in understanding the pathogenesis of autoimmune diseases and developing specific therapies to modulate or block these harmful immune responses.
Challenges in T-cell epitope discovery include the vast diversity of human T-cell receptors, the need for high specificity in epitope mapping, and the complexity of immune responses in different individuals. Accurately predicting and validating epitopes that can universally trigger an effective immune response across different populations also remains a significant hurdle.
Major Histocompatibility Complex (MHC) molecules present peptides on the surfaces of cells and are essential for T-cell recognition. Variability in MHC class I and II molecules among individuals affects which epitopes are presented, influencing the outcome of T-cell epitope discovery and the design of T-cell-targeted therapies.
Yes, T-cell epitope discovery can significantly aid in cancer treatment by identifying tumor-specific antigens that can be targeted by T-cells. This knowledge is used to develop cancer vaccines and adoptive T-cell therapies that enhance the immune system's ability to detect and destroy cancer cells.
In allergen-specific immunotherapy, T-cell epitope discovery is crucial for designing treatments that can desensitize the immune system to specific allergens. Identifying T-cell epitopes in allergens enables the creation of therapies that modulate T-cell responses, potentially leading to long-term tolerance to allergens.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
