As research experience accumulating in the past couple of years and the techniques developed by our excellent research team in epitope mapping, Creative Biolabs offers the unique MHC-peptide binding assay service for our clients.
This technique is a novel approach to rapidly identify T cell epitopes with short turnaround time. It contains three major steps: the detection of MHC-peptide binding assays, followed by rate assays and cell assays to validate the high MHC-affinity peptides. Peptides used for this assay can be provided by clients or synthesized by our peptide library platform.
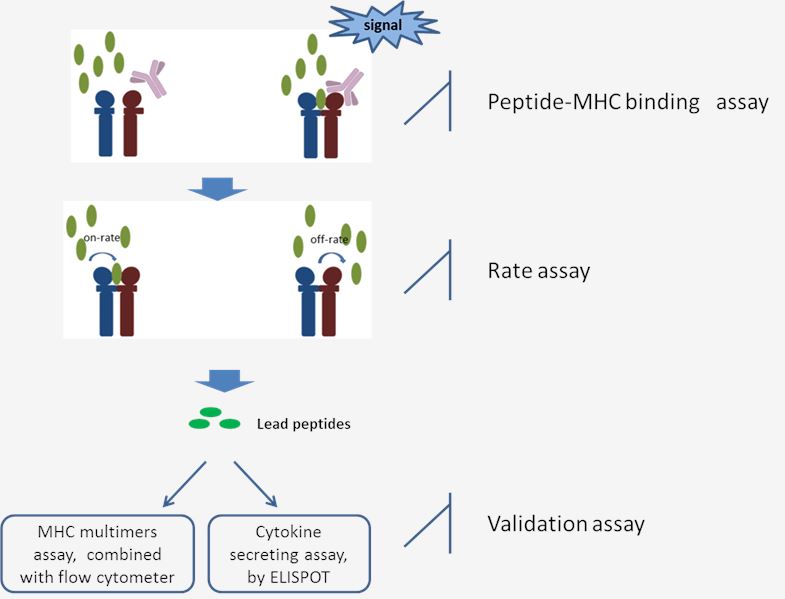 Fig 1. The workflow of MHC-Peptide Binding Assay.
Fig 1. The workflow of MHC-Peptide Binding Assay.
Binding assay: The binding of each peptide with MHC molecules aims to measure the capability of candidate peptides to stabilize the structure of MHC-peptide complex, which is reflected by the distinct signal. Only when the peptides could bind to MHC and stabilize the conformation will the signal be released. Each candidate peptide achieves a score relative to the positive control peptide (known T cell epitope). And based on corresponding analysis of signal percentage, the lead selections will be chosen for the next step study.
Rate assay: This assay is followed by the MHC-peptide binding assay and targets for quantitative determination of the binding abilities of the selected peptides. Peptides that are presented with enough length have the greater chance to be recognized by T cells. The on-or/and off-rates for the selected peptides are measured at different time points. Comprehensive analysis and achieve a score for the individual peptide. The higher score represents a better T cell epitope candidate.
Validation assay: The strategies above will cut down the number of candidate epitopes for further research. Then cell assays could be committed to detecting whether these selected peptides have the property to evoke relevant T cell responses. Detection of the T cell responses induced by immunogenic peptides keeps two major approaches: determine the proliferation of T cells or the cytokine-secreting by T cells in the presence of potentially immunogenic peptides. T cell proliferation assays are performed by combining with the MHC multimers (tetramers, Pentamers, dextramers) techniques which are detected by flow cytometer. The cytokine secreting of T cells is committed with the ultrasensitive ELISPOT technique to accurately reflect the antigen-specific T cell responses.
Creative Biolabs has years of experience in offering our customer epitope mapping techniques and we are happy to provide the advanced technical support to help our clients find the T cell epitope. If you are interested in our service, please contact us for more information and a detailed quote.
Other optional CreMap™ T cell epitope discovery services:
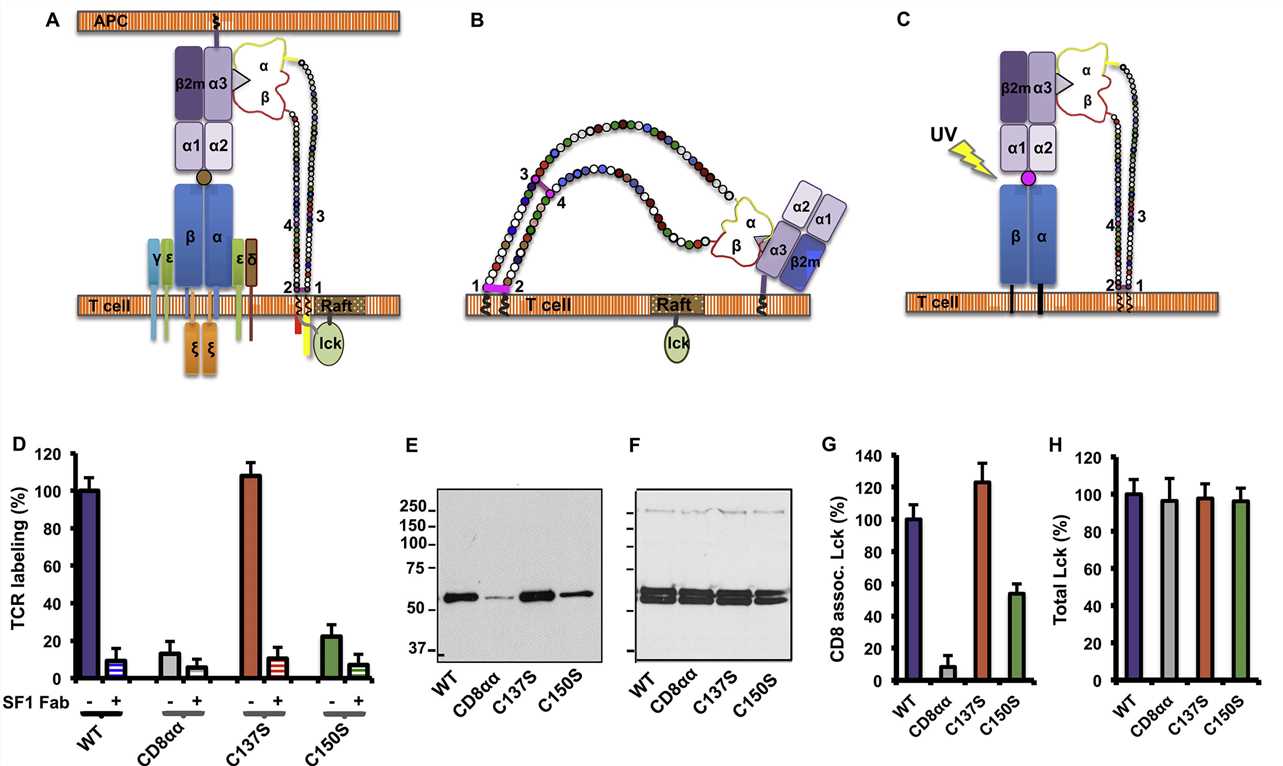 Fig. 2 CD8-pMHC binding in cis negatively regulates CD8 coreceptor function.1,2
Fig. 2 CD8-pMHC binding in cis negatively regulates CD8 coreceptor function.1,2
The article reveals significant insights into the regulation of CD8+ T-cell responses through the interaction of CD8 with MHC-peptide complexes in both cis and trans configurations, highlighting the role of differential binding in modulating T-cell activation. This is mediated by distinct structural interactions and posttranslational modifications, such as a disulfide bond and O-linked glycosylation, which influence the orientation and binding affinity of CD8. The MHC-peptide binding assay is pivotal, demonstrating the alternate binding orientation of CD8 in cis, showing that this interaction can inhibit T-cell activation. This discovery adds complexity to our understanding of T-cell receptor signaling and opens up new possibilities for therapeutic interventions in immune regulation, with potential applications in enhancing immune responses against pathogens or tumors, or in preventing autoimmune responses.
The MHC-peptide binding assay is used to determine the affinity and specificity with which peptides bind to Major Histocompatibility Complex (MHC) molecules. This assay is crucial for understanding T-cell recognition and response, which are central to the immune system's ability to recognize and react to pathogens.
MHC-peptide binding assays can help identify which peptide fragments of proteins will be presented on the cell surface by MHC molecules, a process essential for T-cell immune response. This is critical for developing vaccines, cancer immunotherapies, and studying autoimmune diseases.
The assay typically involves incubating synthetic peptides with purified MHC molecules and measuring the stability of the MHC-peptide complex. Stability is often assessed through techniques like fluorescence polarization, competitive inhibition assays, or surface plasmon resonance, indicating the binding strength and duration.
Both Class I and Class II MHC molecules can be used in binding assays. Class I molecules generally present peptides to CD8+ T cells, while Class II molecules present to CD4+ T cells. The choice of MHC class depends on the specific immune response being studied.
These assays can predict immune responses by identifying peptide epitopes that bind effectively to MHC molecules, suggesting their potential to trigger T-cell activation. However, actual immune response effectiveness can depend on other factors like antigen processing and T-cell receptor variability.
In vaccine development, MHC-peptide binding assays are used to identify peptide sequences that bind strongly to MHC molecules, making them good candidates for vaccine antigens. These peptides can elicit a robust immune response, crucial for effective vaccination.
Results are typically interpreted by comparing the binding affinities of different peptides to MHC molecules. Higher binding affinity indicates a stronger and potentially more effective interaction with T cells, suggesting a greater likelihood of immune activation.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
