Staffed by dedicated scientists with combined knowledge and expertise in antibody engineering and protein X-ray crystallography, Creative Biolabs has extensive experience in crystallizing antibody-antigen complexes and resolving their 3D structures. Based on our extensive knowledge, we are fully equipped to reach out to our clients who may have the problem or difficulty dealing with epitope mapping.
An epitope is the portion of an antigen recognized by the immune system, especially by an antibody. The determination of the amino acid sequence of epitopes is drawing much interest in broad areas such as vaccines and therapeutics. X-ray crystallography of an antibody-antigen complex is the gold standard approach, also the most unambiguous method for epitope mapping. Taking advantage of the interatomic spacing of most crystalline solids, it provides a high-resolution structure of the complex and the molecular details of the interaction are defined as well. Based on the structure and interaction with the antibody, epitopes could be categorized into two types, linear epitopes, and conformational epitopes. X-ray co-crystallography allows for both linear and conformational epitope mappings. By co-crystallography the monoclonal antibody with its respective antigen, the epitope can be defined immediately with high accuracy.
By far, X-ray crystallography is the most precise method for characterizing epitopes. The most significant and essential part of this method is crystallography. It is a process where atoms or molecules are highly organized into a structure called crystal. The principle of crystallization is the fact that solute should be soluble in a suitable solvent at high temperature. A change of these conditions to a state where the solubility is lower leads to the formation of a crystalline solid. One of the most efficient ways for crystallization is hanging drop crystallization. The samples and the reagents are in contact with a siliconized glass surface. This method is cost-effective and has easier access to crystals compared to other methods.
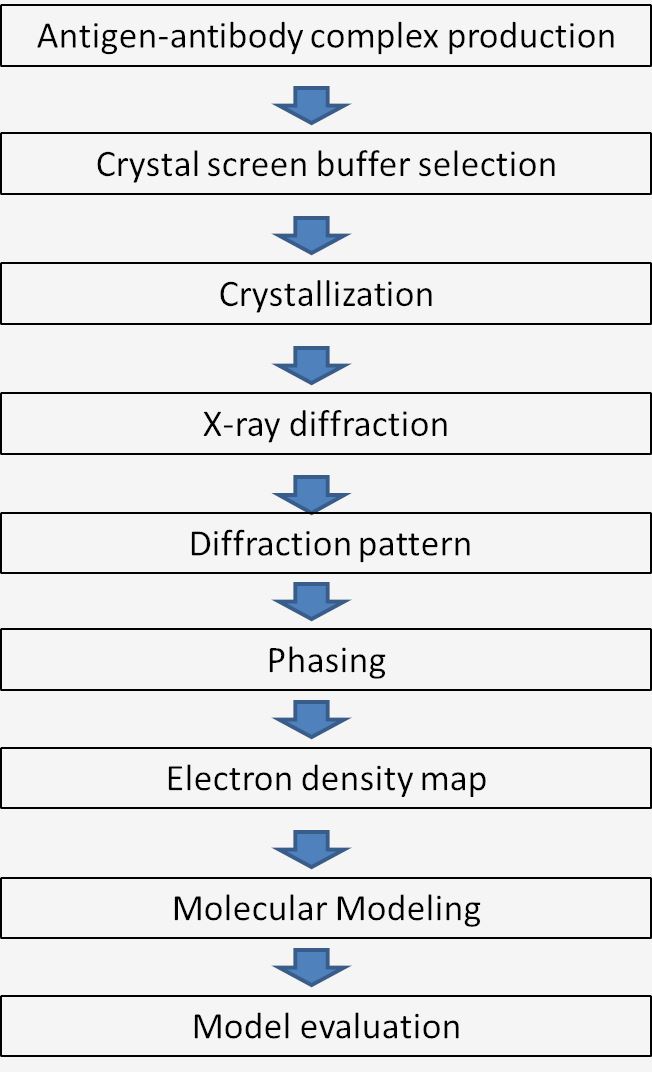 Fig.1 Workflow of X-ray crystallography mechanism.
Fig.1 Workflow of X-ray crystallography mechanism.
Creative Biolabs has accumulated extensive experience in mapping epitopes recognized by the antibodies. If you are now doing research on antigen-antibody complex, trying to figure out epitopes on the antigen, or if you are particularly interested in our X-ray crystallization platform, please feel free to contact us and a formal feedback will be sent back as soon as possible.
Other optional CreMap™ B cell epitope mapping services:
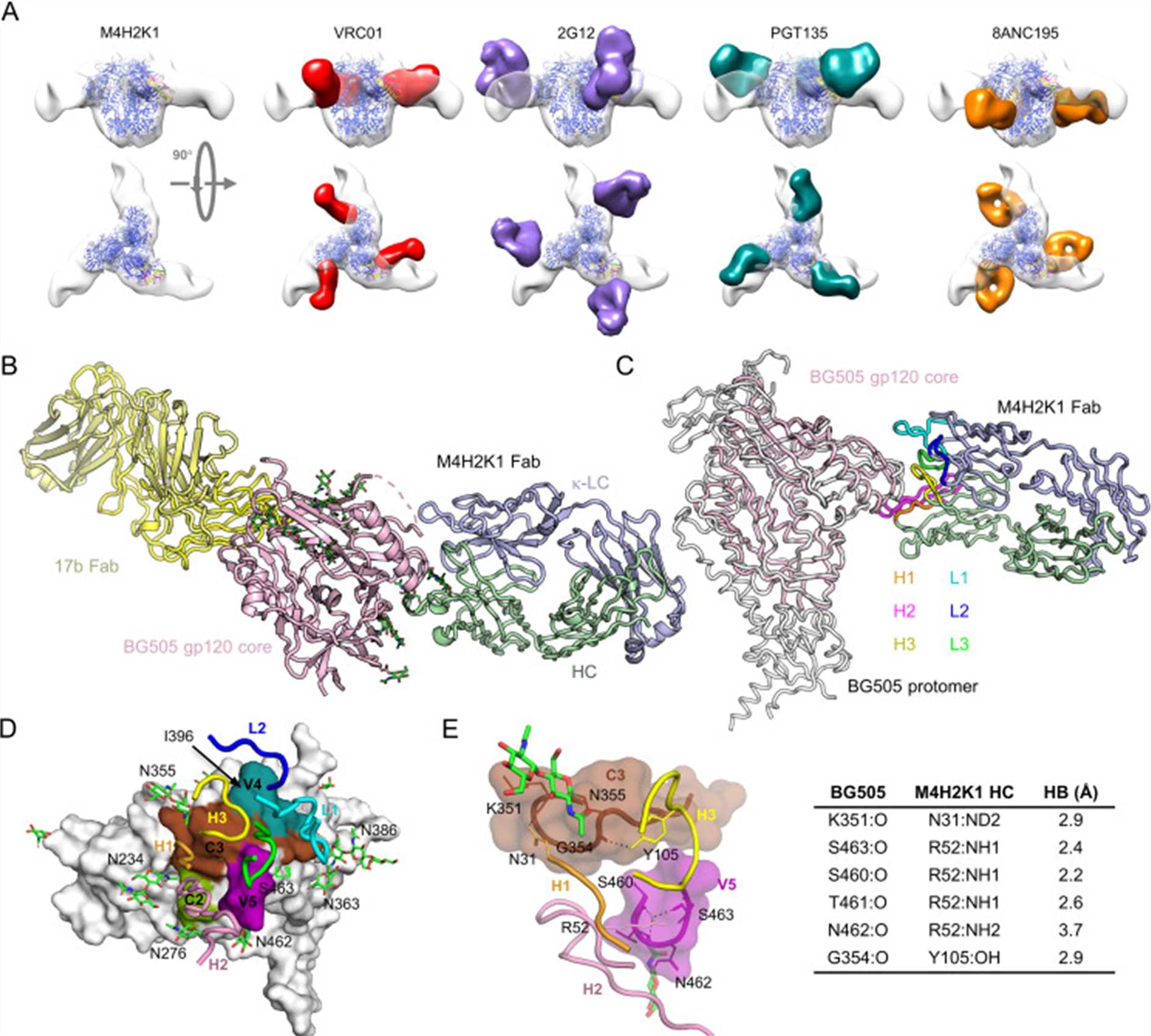 Fig. 2 Structural epitope mapping of M4H2K1 on HIV-1 Env. (Sonu Kumar, 2021)
Fig. 2 Structural epitope mapping of M4H2K1 on HIV-1 Env. (Sonu Kumar, 2021)
The study provides insights into the immunogenicity of gp41-stabilized HIV-1 envelope trimers and nanoparticles, demonstrating the isolation of mouse and rabbit neutralizing antibodies (NAbs) that recognize specific glycan epitopes on the BG505 envelope variant. These NAbs were characterized using techniques such as electron microscopy and X-ray crystallography. X-ray crystallography enabled the precise determination of the three-dimensional structure of the antibody-antigen complexes at high resolution. This allowed the researchers to map the epitopes recognized by the NAbs, identifying interactions at the atomic level, which is crucial for understanding the mechanism of neutralization and guiding the design of vaccines and therapeutic antibodies targeting similar mechanisms in HIV and other pathogens.
Epitope mapping by X-ray crystallography involves determining the structure of an antigen in complex with an antibody at atomic resolution. This method allows scientists to visualize the precise interactions between the antibody and antigen, identifying the specific amino acids involved in binding.
X-ray crystallography provides a detailed view of how antibodies bind to antigens. By crystallizing the complex and analyzing the diffraction patterns produced by X-rays, researchers can map the exact location and nature of the binding site, or epitope, on the antigen, which is crucial for understanding immune recognition and response.
The main advantage of X-ray crystallography is its ability to provide precise, atomic-level structural data. This clarity allows researchers to understand the detailed mechanism of antibody-antigen interactions, which is essential for the rational design of vaccines and therapeutic antibodies.
X-ray crystallography can map epitopes on large antigens, although it can be challenging. The technique is often complemented by other methods like cryo-electron microscopy for very large complexes. However, for most protein antigens, X-ray crystallography is sufficiently powerful to provide detailed epitope maps.
Samples required for X-ray crystallography include highly purified antigen and antibody (or its fragment, such as Fab or Fv), which must be capable of forming a stable complex. The samples should crystallize to form a lattice that diffracts X-rays effectively, allowing the determination of the structure.
X-ray crystallography offers more detailed structural information than techniques like ELISA or mass spectrometry. Unlike these biochemical methods, crystallography provides a visual map of contact points at the atomic level. However, it requires more sample preparation and can be more time-consuming and technically demanding.
The main limitations include the need for large quantities of pure, stable protein that can form crystals, and the technical challenge of obtaining and interpreting high-quality crystal structures. Some protein complexes may be difficult to crystallize, and dynamic interactions may not be observable due to the static nature of crystal structures.
The time required can vary widely, from weeks to months or even longer, depending on the complexity of the antigen-antibody interaction, the ease of crystallization, and the resolution of structural data required. Preparing high-quality crystals and solving the structure are the most time-consuming steps.
Antigens and antibodies that can stably interact and form high-quality crystals are best suited for X-ray analysis. Smaller, less flexible molecules generally crystallize more readily. Antibodies or fragments that retain high affinity for their antigens and do not require post-translational modifications are ideal.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
