Creative Biolabs helps to figure out epitopes by providing mapping services with Surface Plasmon Resonance (SPR) technology that is quite useful in the evaluation of antigen-antibody interaction, affinity measurement, competition assays, ligand fishing, ADME (adsorption, distribution, metabolism, and excretion) characterization.
SPR is the resonant oscillation of conduction electrons at the interface between negative and positive permittivity material stimulated by incident light. SPR allows for the analysis of the association and dissociation rate constants and modeling of biomolecular interaction kinetics. For epitope mapping analysis, the antigen is typically immobilized on a sensor chip surface, and the antibody flows through a microfluidic system in contact with the chip surface. SPR measures in real time the changes in mass bound to the sensor chip. It detects the changes in refractive index of the surface layer of a solution in contact with the sensor chip that is caused by a variation of the mass on the sensor chip surface. SPR immunoassay resembles the concept of the ELISA technique, but it is label-free, eliminating the requirement for reporter molecules or tags which could be time-consuming and even alter the molecular interaction.
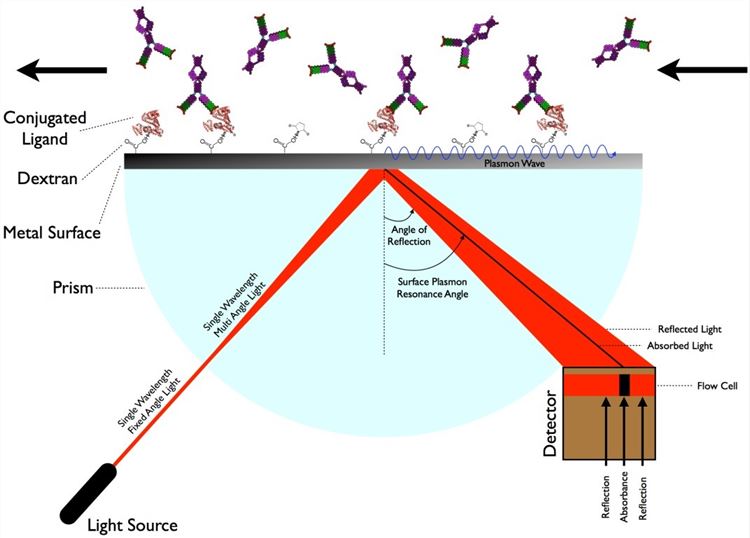 Fig.1 The schematic of the surface plasmon resonance measurement.
Fig.1 The schematic of the surface plasmon resonance measurement.
Surface plasmon resonance (SPR) is a label-free optical sensing technique to characterize binding between a soluble ligand and a surface-immobilized receptor.
SPR is a powerful method for characterizing the epitopes on the antigen.Scientists from Creative Biolabs have been working on developing and optimizing our SPR platform to better serve growing demands of customers around the world. If you are trying to figure out epitopes on the antigen, or if you are interested in our platform, please feel free to contact us by sending E-mail. A formal feedback will be sent back as soon as possible.
Other optional CreMap™ B cell epitope mapping services:
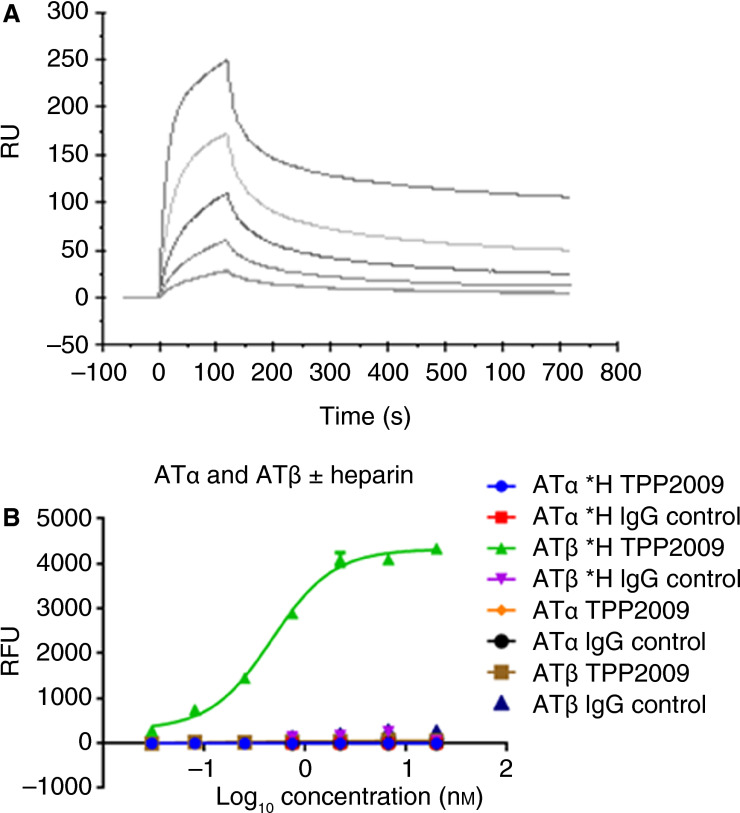 Fig. 2 TPP2009 specifically binds antithrombin III (AT)β in a heparin‐dependent manner. (Y. Jin, 2016)
Fig. 2 TPP2009 specifically binds antithrombin III (AT)β in a heparin‐dependent manner. (Y. Jin, 2016)
The study utilizes Surface Plasmon Resonance (SPR) for epitope mapping to specifically investigate the interaction of a novel conformation-specific antibody, TPP2009, with Antithrombin IIIb (ATb) when bound to heparin. This research demonstrates that TPP2009 can selectively bind to ATbH and effectively block ATb-mediated anticoagulation, suggesting a targeted therapeutic approach for conditions involving excessive clotting. The results show that TPP2009 specifically targets a unique conformational epitope on ATbH, significantly reducing its anticoagulant effect and thereby enhancing coagulation in plasma. This selective inhibition is pivotal in developing interventions that manipulate hemostasis precisely at sites of vascular injury, potentially providing a novel strategy for managing thrombotic diseases.
Epitope mapping by SPR is a method that identifies the specific parts of an antigen that antibodies recognize and bind to. It involves the real-time monitoring of binding events between an antigen and antibody, without the need for labeling, providing detailed insights into the molecular interactions at the epitope level.
In SPR epitope mapping, an antigen is typically immobilized on a sensor chip and various antibodies are flowed over the surface. SPR measures changes in the refractive index near the sensor surface, which occur when antibodies bind to the antigen. This data helps identify which parts of the antigen are involved in binding.
SPR allows real-time monitoring of binding interactions, requires minimal sample preparation, and provides quantitative data on binding kinetics and affinity. It can also be used to study interactions under near-physiological conditions, making it highly relevant for therapeutic antibody development.
SPR is sensitive enough to differentiate between closely related epitopes. By analyzing the binding kinetics and affinity of antibodies to specific antigen regions, SPR can distinguish subtle differences in antibody-antigen interactions, even among closely spaced or similar epitopes.
SPR can analyze a wide range of biological samples including proteins, peptides, small molecules, and even cells. For epitope mapping, the antigens and antibodies used are typically purified proteins or peptides, which are stable under the conditions used in the SPR experiments.
Modern SPR instruments are equipped with multiple flow channels and automated sample handling systems, enabling high-throughput analysis. This allows for simultaneous testing of multiple antibodies against a single antigen or screening of several antigens in a single experiment.
SPR can indirectly detect conformational changes in antigens during antibody binding through changes in the sensorgram, which may indicate complex formation or dissociation kinetics that deviate from expected patterns. Additional experiments, such as mass spectrometry, might be necessary to confirm these findings.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
