Bio-better Glucocerebrosidase Glycoengineering Service
Efficient Glucocerebrosidase (GCD) Glycoengineering Service at Creative Biolabs
GCD is an important lysosomal enzyme whose function is to hydrolyze glucocerebroside and break it down into glucose and ceramidoglucolipid. GCD is mainly used to treat a single-gene disease called Gaucher disease (GD). This treatment method is called "enzyme replacement therapy" (ERT), which achieves the therapeutic goal by replacing the defective gene product GCD. GCD was originally extracted from the human placenta and processed through a series of steps to obtain N-glycans containing terminal mannose residues, thereby targeting the mannose receptor (MR) on macrophages to treat GD. However, the source of the placenta is limited, so there is an urgent need to develop GCD from other sources. Based on this, Creative Biolabs provides professional bio-better glucocerebrosidase glycoengineering services.
-
Mammalian systems-based bio-better GCD glycoengineering service
GCD consists of 497 amino acids and contains 5 N-glycosylation sites. Its structure contains complex oligosaccharide chains and high mannose. This glycosylation pattern is crucial for the production of active proteins. We have developed stable cell lines (such as the Chinese hamster ovary system (CHO)) to express GCD with mannose-terminal N-glycan. The main steps are as follows:
-
Construction of expression vector: We clone the GCD gene into an expression vector.
-
Transfection of CHO cells: We use appropriate transfection methods to introduce the constructed expression vector into CHO cells and select stable cell lines.
-
Cell expansion: We use cell culture technology to expand the number of stable cell lines.
-
Protein expression: We optimize cell culture conditions and use mannosidase I inhibitors in the culture medium to produce high mannose-type glycans. Mannose-terminated glycans better bind to MR, thereby targeting the protein to macrophages.
-
Protein purification: We use affinity chromatography, ion exchange chromatography, and other technologies to purify the expressed protein.
-
Plant systems-based bio-better GCD glycoengineering service
We construct an expression plasmid containing the GCD gene, and introduce the constructed expression vector into carrot cells through Agrobacterium-mediated transformation. Moreover, we provide appropriate culture conditions, such as culture medium, temperature, light, etc., to promote cell growth and expression of enzymes with terminal mannose residues. GCD is then homogenized and extracted with sodium phosphate (pH 7.2), and is purified and analyzed.
Published data
GD is an inherited lysosomal storage disorder caused by a lack of functional enzyme β-glucocerebrosidase (GCase), which is derived from mutations in the GBA1 gene. Currently, ERT is considered the most effective treatment for type 1 GD. The key to this approach is to infuse patients with exogenous recombinant GCase, which contains a terminal mannose N-glycan structure that ensures the effective entry of the recombinant GCase into macrophages through the mannose receptor. In this study, the authors developed a protein production system to produce recombinant GCase with mannose N-glycan structures in wild-type (WT) and glycoengineered Nicotiana benthamiana plants (ΔgntI) through Agrobacterium-mediated transient expression. The recombinant GCase was then purified and its sugar structure and enzyme activity were analyzed. In addition, the authors demonstrated that this glycoengineered plant system may also be suitable for the production of other proteins, especially mannose receptor-targeted proteins for therapeutic purposes.
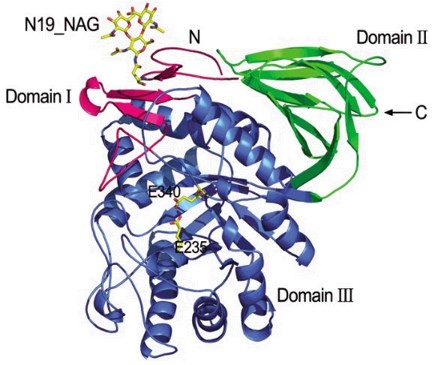 Fig.1 LC-MS spectra of recombinant GCase produced in WT and ΔgntI.1
Fig.1 LC-MS spectra of recombinant GCase produced in WT and ΔgntI.1
Advantages
-
We have developed a comprehensive Glycoengineering Platform to produce Therapeutic Proteins with custom-modified glycan patterns.
-
We provide high-efficiency and high-quality GCD glycoengineering service and provide custom service according to client needs.
-
We have strong scientific research capabilities and provide clients with one-stop GCD glycoengineering solutions.
Application
-
By performing glycoengineering services on GCD, its pharmaceutical properties and biological activities may be improved, which has potential value for the treatment of related genetic metabolic diseases such as Gaucher disease.
-
Bio-better GCD is used to produce more effective and stable biologics, improving production efficiency and product quality.
-
Bio-better GCD serves as a key component in cellular research to develop therapeutic strategies for various neurodegenerative or other diseases.
Creative Biolabs has professional teams, technical strength, and advanced glycoengineering technology platforms, and provides efficient GCD glycoengineering service according to client needs. Please feel free to contact us if you would like to acquire detailed service.
FAQ
Q1: Why do we need to perform glycoengineering services on GCD?
A1: Glycoengineering services on GCD are performed to improve its drug properties and biological activity, especially in the treatment of genetic metabolic diseases such as GD. The improved GCD can bind to mannose receptors on macrophages more effectively, thereby improving the therapeutic effect. In addition, through expression in cell systems (such as CHO cells or plant systems), the limitations of traditional sources (such as human placenta) are overcome, production efficiency and product quality are improved, and the sustainable supply of products is increased. These optimizations all help to provide more effective and economical "enzyme replacement therapy" for the treatment of GD and other related genetic metabolic diseases.
Q2: What is the difference between GCD produced in plant systems and CHO systems?
A2: GCD produced in plant systems and CHO systems may differ in glycosylation patterns, catalytic efficiency, and immunogenicity. Especially in terms of glycosylation, plant systems will contain plant-specific glycosylation structures, while CHO systems are closer to the glycosylation patterns of mammalian cells, so that enzymes produced in CHO systems may perform better in mammals (such as half-life and immunoreactivity). In addition, these systems have their advantages and disadvantages in practical applications such as production efficiency, cost, and scalability.
Q3: How to purify the expressed GCD protein?
A3: In the purification process of GCD protein, affinity chromatography, and ion exchange chromatography are usually used. These methods can effectively separate and extract the target protein, ensuring high-purity GCD to meet the needs of subsequent therapeutic uses.
Customer Review
Produced GCD with Higher Activity
“The stable cell line development process went very smoothly. The use of the CHO cell system made the expression and purification of GCD more efficient, which was the key to the success of our project. The Bio-better GCD produced through this service showed higher biological activity in our cell studies, providing new possibilities for the treatment of GD diseases.”
Professional Technology And High-quality Products
“The team of Creative Biolabs excelled in GCD glycoengineering services. From constructing expression vectors to protein purification, every step was strictly controlled to ensure the high quality of the final product.”
Reference
-
Uthailak, Naphatsamon, et al. "Transient production of human β-glucocerebrosidase with mannosidic-type N-Glycan structure in glycoengineered Nicotiana benthamiana plants." Frontiers in Plant Science 12 (2021): 683762. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services


 Fig.1 LC-MS spectra of recombinant GCase produced in WT and ΔgntI.1
Fig.1 LC-MS spectra of recombinant GCase produced in WT and ΔgntI.1



