High Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography (HPLC) is a sensitive analytical technique that is widely used for the isolation and analysis of both glycoproteins and their derived carbohydrates. Based on this technique, Creative Biolabs is committed to offering a wide range of glycoprotein analysis service for customers all over the world.
Glycoproteins Analysis Based on HPLC
HPLC is a form of column chromatography that pumps a sample mixture or analyte in a solvent (called the mobile phase) at high pressure through a column with chromatographic packing material (stationary phase). The sample is carried by a moving carrier gas stream of helium or nitrogen. HPLC has the ability to separate and identify compounds that are present in any sample that can be dissolved in a liquid in trace concentrations as low as parts per trillion. The main advantages of HPLC include excellent resolution, ease of use, the generally high recoveries, the excellent reproducibility of repetitive separations, and the high productivity in terms of cost parameters.
HPLC is also one of the most commonly used methods for the isolation and analysis of both glycoproteins and their derived carbohydrates. Reversed-phase HPLC (RP-HPLC) has become a commonly used method for the analysis and purification of peptides, proteins, and glycoproteins. The RP-HPLC experimental system usually includes an n-alkylsilica-based sorbent. Complex mixtures of peptides and proteins can be separated and low picomolar amounts of resolved components can be collected by using modern instrumentation and columns. Subsequently, separated fractions can be used for further analysis of carbohydrates and amino acids. Besides, a new HPLC method, HPLC-pulsed amperometric detection (PAD), which bypasses the derivatization steps by using pulsed electrochemical detection on gold electrodes, has been developed. HPLC-PAD has been used successfully in resolving and quantitating the constituent monosaccharides released by acidic hydrolysis of glycan chains and for resolving N-linked oligosaccharides separated by enzyme digestion.
 Fig. 1 Preparative HPLC system. Distributed under Open Access license CC BY-SA 3.0, from Wiki, without modification.
Fig. 1 Preparative HPLC system. Distributed under Open Access license CC BY-SA 3.0, from Wiki, without modification.
HPLC Analysis Services
Creative Biolabs has successfully developed an advanced and sensitive platform for glycoprotein/glycopeptide analysis based on HPLC technique. Based on RP-HPLC, complex mixtures of glycoproteins can be separated efficiently and the separated carbohydrates and amino acids can be analyzed by combined with other detection technologies. Based on HPLC-PAD, monosaccharides released by acidic hydrolysis of glycan chains are quantitatively analyzed. Besides, we also provide a custom service for global clients to meet their special purposes.
Features of Our Services
-
Excellent technical team
-
High-quality analysis services
-
Advanced technique platforms
-
Whole-process technical trace
Professional scientists in Creative Biolabs can help our client to design the optimal analytical protocol and attain their interested experimental data to meet their special project requirement. We have established a powerful HPLC-based analytical platform which is applied for Glycan Profiling, Glycomic Profiling, and Glycosylation Site Mapping. If you are interested in our HPLC analysis services, please feel free to contact us for more details.
Published data
Abnormal glycosylation is common in the development of cell tumors. The levels of O- or N-sugars in the serum of healthy individuals and patients with different cancers are different. These sugars may play a key role in the clinic as biomarkers. In this study, the authors developed and validated an HPLC method for analyzing serum monosaccharide levels in patients with endometrial cancer (EC) and healthy controls. First, they collected venous blood samples from patients and controls and prepared serum specimens to be analyzed by centrifugation and other steps. Subsequently, they selected L-rhamnose as the internal standard, prepared standard curve working solutions of 8 monosaccharides, and labeled these 8 monosaccharides with 1-phenyl-3-methyl-5-pyrazolone (PMP), including D-glucose, D-mannose, D-xylose, D-galactose, glucosamine, glucuronic acid, galactosamine, and L-fucose. Next, they optimized the HPLC chromatographic conditions, separated and detected each monosaccharide, and verified the sensitivity, limit of quantification, accuracy, stability, linearity, and other parameters of the detection method. Finally, they used the verified HPLC method to quantitatively analyze the monosaccharides in different serum samples. The results showed that under the current HPLC chromatographic conditions, each monosaccharide (Figure A) and the internal standard (Figure B) could be well separated. The monosaccharide level in the patient's serum (Figure D) was significantly higher than that in the control group (Figure C). This showed that the HPLC method developed by the researchers was a reliable, simple, and reproducible monosaccharide analysis method, which was suitable for the analysis of clinical samples and had potential value as an EC screening biomarker.
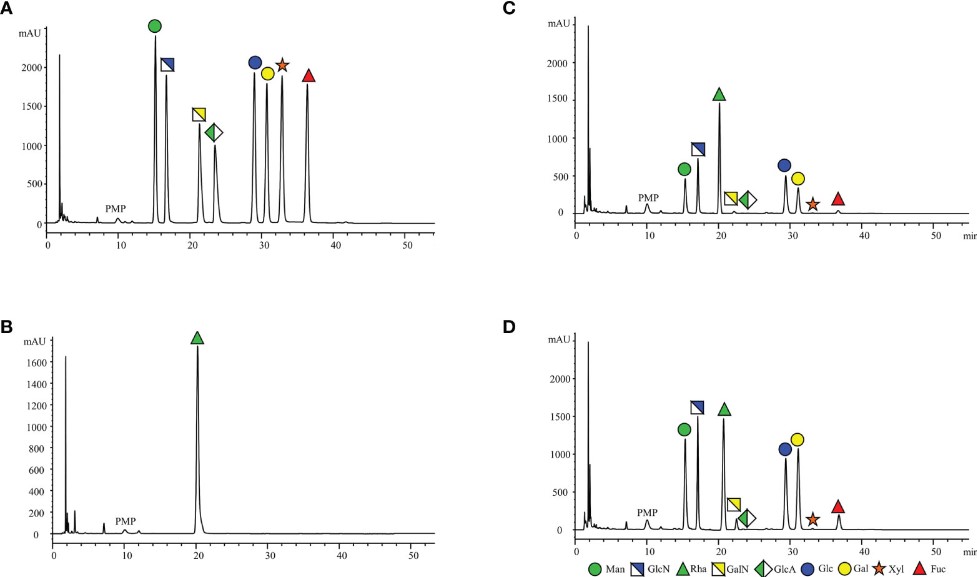 Fig.2 Representative HPLC chromatograms of PMP-labeled monosaccharides.1
Fig.2 Representative HPLC chromatograms of PMP-labeled monosaccharides.1
FAQs
Q1: How to separate peptides, proteins, and glycoproteins using RP-HPLC?
A1: In the RP-HPLC system, the sample is pumped into a column filled with n-alkyl silica gel adsorbents by a high-pressure pump. This adsorbent effectively separates complex peptide and protein mixtures through hydrophobic interactions. We then use mobile phases composed of different proportions of organic solvents (such as acetonitrile) to achieve elution and separation of different components in the sample.
Q2: What specific HPLC analysis projects do you provide?
A2: We provide a variety of HPLC analysis services, such as glycosylation site mapping, glycan and glycomic profiling, etc. In addition, clients can also customize analysis services according to their own needs, such as glycoform analysis of specific proteins, quantitative monosaccharide analysis, etc.
Q3: What kind of sample pretreatment is required for HPLC analysis?
A3: The sample pretreatment steps depend on the specific analysis content. For example, the analysis of glycoproteins may involve protein separation and purification, deglycosylation steps, enzymatic hydrolysis, etc. For HPLC-PAD analysis, acid hydrolysis of the sample is usually required to release monosaccharides. Additionally, the sample needs to be dissolved in an appropriate solvent and filtered to remove particulate matter to ensure that the sample does not overload the HPLC column.
Customer Review
Excellent Separation Results
"Creative Biolabs uses HPLC analysis technology to separate glycoproteins in complex mixtures. Through RP-HPLC, not only high-resolution separation is achieved, but also reliable and efficient analysis of subsequent carbohydrates and amino acids is performed. Their analysis services are highly recommended."
Accurate Quantitative Analysis
"Creative Biolabs uses HPLC-PAD technology to perform quantitative analysis of monosaccharides obtained by acid hydrolysis, and the results are very accurate. This analysis method not only eliminates the tedious derivatization step but also greatly improves the sensitivity of the detection."
References
-
Chen, Yulong, et al. "Determination of monosaccharide composition in human serum by an improved HPLC method and its application as candidate biomarkers for endometrial cancer." Frontiers in Oncology 12 (2022): 1014159. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig. 1 Preparative HPLC system.
Fig. 1 Preparative HPLC system.  Fig.2 Representative HPLC chromatograms of PMP-labeled monosaccharides.1
Fig.2 Representative HPLC chromatograms of PMP-labeled monosaccharides.1

