Custom Synthesis Services
Recombination therapeutic proteins are most glycosylated and the protein glycosylation may have an effect on the structure and function of therapeutic proteins. With the highly specialized equipment and experienced expert staff, Creative Biolabs can provide a full range of custom synthesis services, including glycans synthesis, glycopeptide synthesis, and glycoprotein synthesis, with the highest quality and the most competitive price for global clients.
Background of Protein Glycosylation
Protein glycosylation is a common posttranslational modification that plays a very important role in various biological processes including cell-cell recognition, immune response, and cellular signal transduction. Approximately more than 50% of proteins undergo glycosylation modification. Importantly, more than 60% of those recombinant therapeutic proteins are glycosylated and glycosylation pattern has an effect on the efficacy, half-life, stability, and safety of therapeutic proteins. In order to investigate the relationship between structure and function of glycoproteins, it is required to synthesize homogeneous glycoproteins with defined oligosaccharide side chains.
Unlike nucleic acid and protein, biosynthesis of glycopeptide or glycoprotein is non-template. Hence, glycoproteins always exist as heterogeneous mixtures with different glycan structures and it is difficult to synthesize homogenous glycoproteins in vitro. Over the past years, different strategies have been developed for obtaining the homogenous glycoproteins including chemical and chemoenzymatic methods. This not only helps to investigate the structure and function of glycoproteins but also facilitates the development of therapeutic recombination glycoproteins.
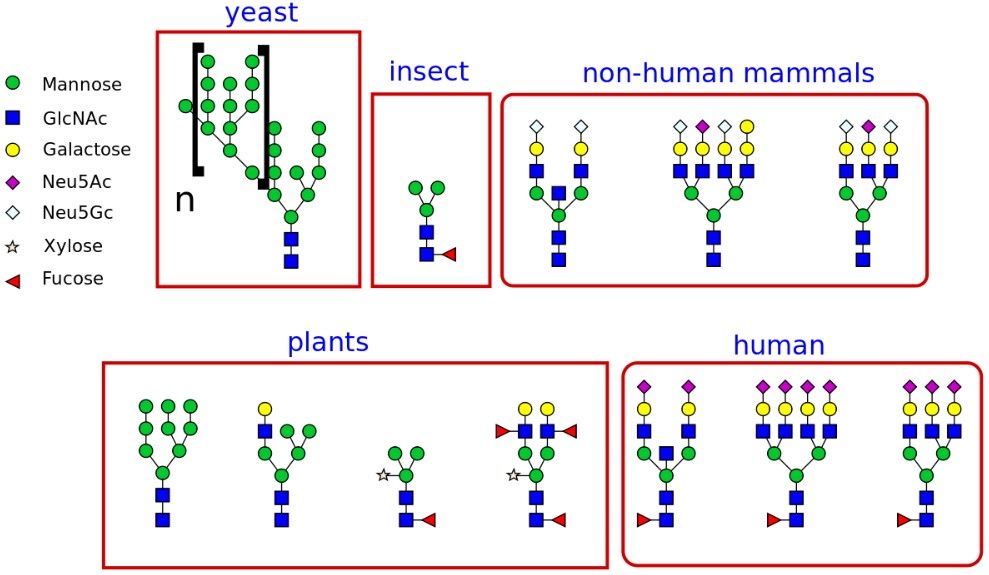 Fig.1 The different types of glycans produced in different organisms. Distributed under Open Access license CC BY-SA 3.0, from Wiki, without modification.
Fig.1 The different types of glycans produced in different organisms. Distributed under Open Access license CC BY-SA 3.0, from Wiki, without modification.
Approaches of Custom Synthesis
Chemical synthesis is a common method that utilizes chemical link coupling two substances. This method has been applied for glycopeptide and glycoprotein synthesis. Currently, there have two strategies for glycopeptides/glycoproteins synthesis. The first is to form a linkage between glycan and protein early to form glycopeptide building blocks that may then be assembled. The second is the construction of the linkage at the later stages of synthesis once the protein scaffold is in place. In addition, chemical method is also used for small oligosaccharide side chains, such as those found in O-linked glycans.
It is known that enzymes exert an essential in the biosynthesis of macromolecule via promoting synthetic rate. During the process of glycosylation, many enzymes such as glycosidases and glycosyltransferases have been found in vivo to promote the production of glycopeptide and glycoproteins. Moreover, these enzymes also function in vitro glycosylation processing. Now a variety of enzymes have been available through isolation from natural sources or by heterologous overexpression in exogenous hosts. Furthermore, these enzymes have been shown to accept saccharides, glycolipids, and glycoproteins as substrates. By using an excess of enzyme, high yielding reactions are possible even if the substrates are poor.
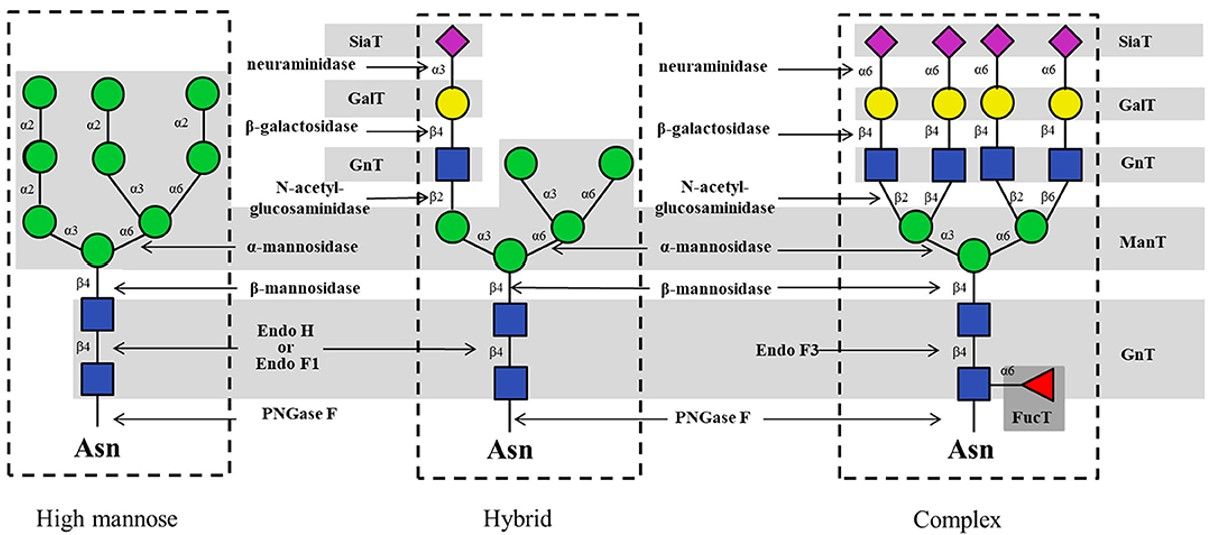 Fig.2 Typical enzymes for enzymatic synthesis of N-glycans.1
Fig.2 Typical enzymes for enzymatic synthesis of N-glycans.1
Custom Synthesis Services in Creative Biolabs
Creative Biolabs is an omnibearing service provider of custom synthesis. Our professional experts have extensive experience in glycoprotein synthesis. We are committed to offering the most comprehensive custom synthesis services with the highest quality and most competitive price to our worldwide clients. In order to meet every specific need of our clients’ research and project development, our scientists are pleased to tailor the best-fit approach to provide a comprehensive custom synthesis service. Our services include:
Features of Our Custom Synthesis Services
-
High-quality and low-cost custom synthesis service
-
Professional technical team specialized in the custom synthesis
-
Across-the-board technical guidance
-
Rapid and high-quality solutions
Equipped with advanced technologies and professional scientists, we are confident to provide comprehensive and high-quality custom synthesis services. We believe that our high-quality services will help you get through many thorny issues in custom synthesis. Please feel free to contact us for more details.
Published data
As one of the most common post-translational modifications, N-glycosylation affects the biological functions of glycoproteins. The production of homogeneous N-glycans with well-defined structures is fundamental to the comprehensive study of glycoprotein structure and biological roles. This paper introduces us to the progress of research on producing N-glycans using chemoenzymatic methods, including the preparation of sugar donors and substrates, and the characterization of glycosyltransferases involved in the synthesis. The chemoenzymatic method has high stereoselectivity and is efficient for N-glycan synthesis. Several strategies have been developed to synthesize high-mannose-type N-glycans, such as top-down and bottom-up chemoenzymatic strategies. In addition to this, researchers have established several chemo-enzymatic strategies for synthesizing asymmetric N-glycans. The N-glycans synthesized through these have multiple applications, including the construction of glycan microarray, homogeneous glycoprotein synthesis, and so on. It not only simplifies the preparation of homogeneous glycoproteins but also lays the foundation for studying the role and functions of N-glycans in glycoproteins.
N-glycans are a class of structurally diverse oligosaccharide structures. The chemoenzymatic method combines the features of chemical and enzymatic synthesis methods and has some advantages such as mild reaction conditions and high specificity. These methods summarized in this paper provide strong support for the custom synthesis of glycans.
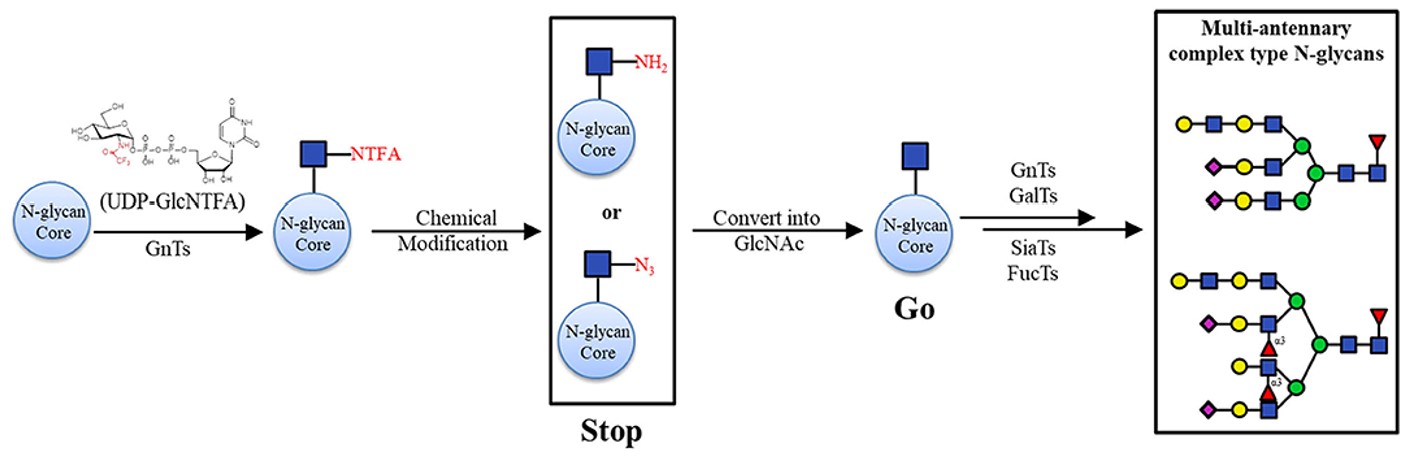 Fig.3 Chemoenzymatic synthesis of asymmetric branching N-glycans.1
Fig.3 Chemoenzymatic synthesis of asymmetric branching N-glycans.1
FAQs
Q1: What are the advantages of chemoenzymatic methods in synthesizing glycans?
A1: Chemoenzymatic methods start with the chemical synthesis of naturally sourced N-glycan precursors or key N-glycan modular structures, followed by enzymatic extension steps to obtain complex N-glycans. The chemoenzymatic approach offers several advantages over chemical synthesis and enzymatic glycosylation for the synthesis of glycans. It offers the flexibility of the chemical approach in addition to the regioselectivity and stereoselectivity of the enzymatic reaction.
Q2: How long does it usually take to complete a custom synthesis project?
A2: Custom synthesis time for glycans, glycopeptides, and glycoproteins vary depending on client requirements and the complexity of the project. After we understand your needs, we provide a proposal and a detailed project schedule to give you a better idea of how long this service takes to perform.
Customer Review
First-class Custom Synthesis Service
"We were impressed by Creative Biolabs' extensive experience in custom synthesizing glycopeptides. Not only did they synthesize high-quality and high-purity glycopeptides, but they also answered some of our questions in this area. Their professionalism was top-notch."
Timely Delivery of Custom Synthesized Products
"Creative Biolabs recently custom-synthesized glycopeptides for our research needs. Not only was the delivery rapid, but the quality of the product was also very high. The high-quality of their deliverables and synthetic reports was recommendable."
References
-
Chao, Qiang, et al. "Recent progress in chemo-enzymatic methods for the synthesis of N-glycans." Frontiers in Chemistry 8 (2020): 513. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 The different types of glycans produced in different organisms.
Fig.1 The different types of glycans produced in different organisms.  Fig.2 Typical enzymes for enzymatic synthesis of N-glycans.1
Fig.2 Typical enzymes for enzymatic synthesis of N-glycans.1
 Fig.3 Chemoenzymatic synthesis of asymmetric branching N-glycans.1
Fig.3 Chemoenzymatic synthesis of asymmetric branching N-glycans.1

