Enzyme-Mediated Saccharification Efficiency Analysis Service
Services Published Data FAQs Customer Review
Reliable Analysis of Enzyme Saccharification Efficiency at Creative Biolabs
Enzyme saccharification efficiency refers to the efficiency of enzymes in hydrolyzing polysaccharide substrates (such as starch, cellulose, etc.) into monosaccharides (such as glucose). Factors such as the hydrolysis rate of the substrate by the enzyme, the concentration of monosaccharides produced, and the activity and stability of the enzyme will all affect the saccharification efficiency. By analyzing the enzyme saccharification efficiency, the most effective reaction conditions and enzyme dosage can be determined, thereby optimizing the production process and improving production efficiency. Moreover, efficient enzymatic hydrolysis can ensure the stability and consistency of product quality, helping to produce high-quality end products. Creative Biolabs has rich experience and a good reputation in the field of enzyme saccharification efficiency analysis, which has provided related services to many clients and achieved remarkable results. We provide custom enzyme saccharification efficiency analysis services according to your needs. In addition, we also provide Enzyme Degradation Efficiency Analysis, Cellulolytic Enzyme, and Amylolytic Enzyme Activity Assessment services.
We perform saccharification efficiency analysis for many types of enzymes, including amylase, cellulase, xylanase, etc. We usually conduct enzyme saccharification efficiency analysis through the following steps.
According to the analysis needs, we design the experimental plan including the control group and the experimental group, and then determine the reaction conditions (temperature, pH, reaction time, etc.).
We will pre-process samples (such as starch, cellulose, etc.) according to actual needs, such as crushing or extraction.
-
Preparation of substrate mixture
We mix the sample with the appropriate substrate in a reaction tube or reaction system and add the enzyme to be tested, making sure to mix evenly. For example, alpha-amylase and glucoamylase can break down starch polymers into glucose monomers, cellulase can break down cellulose into glucose, and xylanase can break down lignocellulose into xylose.
We place the mixed sample under appropriate temperature and pH conditions and maintain it for a period to allow the enzyme to fully act on the substrate mixture so that the hydrolysis reaction occurs. We then terminate the reaction using appropriate methods (e.g., heating/cooling, addition of reagents) to prevent further hydrolysis from occurring. For hydrolyzed samples, we centrifuge, dilute, or otherwise process them for subsequent analysis.
-
Determine product concentration
We use chromatography, mass spectrometry, spectrophotometry, etc. to detect the concentration of monosaccharides produced during the hydrolysis of starch into glucose and cellulose into monosaccharides to evaluate the hydrolysis efficiency of enzymes. These techniques are often used in conjunction with standard curve methods to quantitatively determine the concentration of specific components in the product to evaluate enzyme activity and hydrolysis efficiency.
-
Calculation of enzyme activity and saccharification efficiency
We calculate the enzyme activity per unit time according to the monosaccharide production rate and calculate the enzyme's saccharification efficiency by comparing the monosaccharide production in the test group and the control group.
Through the above steps, we systematically analyze the efficiency of enzymes in the saccharification process, evaluate the role of enzymes, and optimize reaction conditions, thereby helping to achieve more efficient substrate conversion and production processes. If you would like to know more about the details of saccharification efficiency, please contact us and we will respond to your needs promptly.
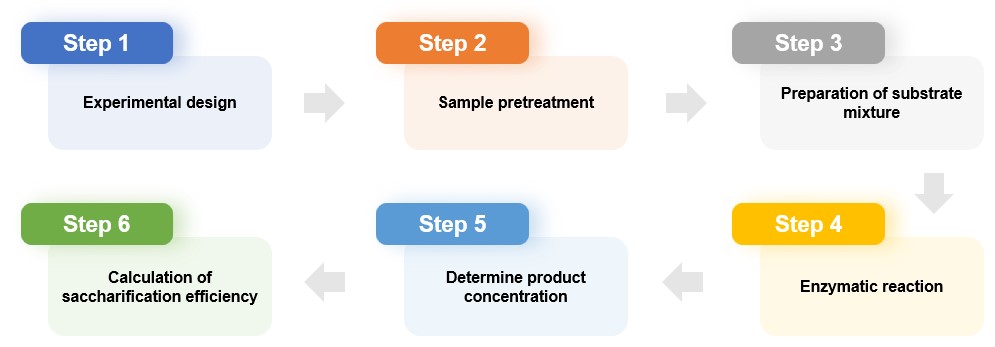 Fig.1 One-stop enzyme saccharification efficiency analysis process.
Fig.1 One-stop enzyme saccharification efficiency analysis process.
Published data
Lignocellulose is an abundant renewable biomass composed of lignin, cellulose, and hemicellulose, and is one of the important sources of liquid fuels and chemicals. In the process of biomass conversion, high-performance enzyme systems play a vital role. In this study, the authors compared the enzymatic activities of three commercial cellulases and studied the hydrolysis performance of these enzymes on different substrates (delignified corncob residue (DCCR) and corncob residue (CCR)) by measuring the glucan conversion efficiency. The results showed that the amount of glucose released in DCCR was higher under lower enzyme dosage conditions (Figure a). With the increase in enzyme dosage, the three enzyme preparations in DCCR showed similar glucan conversion rates (Figures b, c). However, the glucan conversion rates using CCR as substrate were significantly reduced.
 Fig.2 Analysis of glucan conversion during hydrolysis of DCCR and CCR.1
Fig.2 Analysis of glucan conversion during hydrolysis of DCCR and CCR.1
FAQ
Q1: How do you determine the best enzyme for my substrate? How are the source and quality of enzymes ensured?
A1: We select the most suitable enzyme type according to your substrate type and requirements. Enzymes are sourced from reliable suppliers and we conduct quality verification to ensure that their activity and stability meet requirements.
Q2: How much does the enzyme saccharification efficiency analysis service cost? Are there different service packages available?
A2: We will determine the fee according to factors such as the complexity of the experiment, resources required, and time cost. In addition, we provide a variety of service packages for clients to choose from.
Q3: Do you provide technical support and consulting services during the experiment? How do you interpret the results after the experiment is completed?
A3: We provide full technical support and consulting services and are ready to answer your questions at any time. Once the experiment is complete, we will professionally interpret the results and discuss the meaning of the experimental data with you.
Customer Review
Professional Analysis and Advice
"The enzyme saccharification efficiency analysis service at Creative Biolabs helped us identify key factors influencing saccharification efficiency, leading to significant improvements in our biochemical processes. We are grateful for the valuable insights provided."
Reliability and Accuracy
"The Creative Biolabs's enzyme saccharification efficiency analysis service stands out for its reliability and accuracy. The comprehensive analysis report gave us a deep dive into our enzymatic processes and enabled us to make informed decisions for optimization."
Reference
-
Song, Wenxia, et al. "Proteomic analysis of the biomass hydrolytic potentials of Penicillium oxalicum lignocellulolytic enzyme system." Biotechnology for Biofuels 9 (2016): 1-15. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 One-stop enzyme saccharification efficiency analysis process.
Fig.1 One-stop enzyme saccharification efficiency analysis process.
 Fig.2 Analysis of glucan conversion during hydrolysis of DCCR and CCR.1
Fig.2 Analysis of glucan conversion during hydrolysis of DCCR and CCR.1

