T Cell Glycoengineering Service
Services Published Data FAQs Customer Review
Professional T Cell Glycoengineering Service at Creative Biolabs
T cells are a class of immune cells that play a vital role in the body's immune system. The purpose of performing glycoengineering services on T cells is to change the glycosyl structure on the surface of T cells, thereby regulating the activity, functions, and ability of T cells to target specific targets, which helps to enhance the effect of immunotherapy and may improve the treatment of diseases such as cancer. treatment effect. Creative Biolabs has an advanced Cell Line Glycoengineering technology platform, professional scientific research teams, and rich project experience. We focus on providing clients with high-quality Immune Cell Glycoengineering Services, including NK Cell and T cell glycoengineering services.
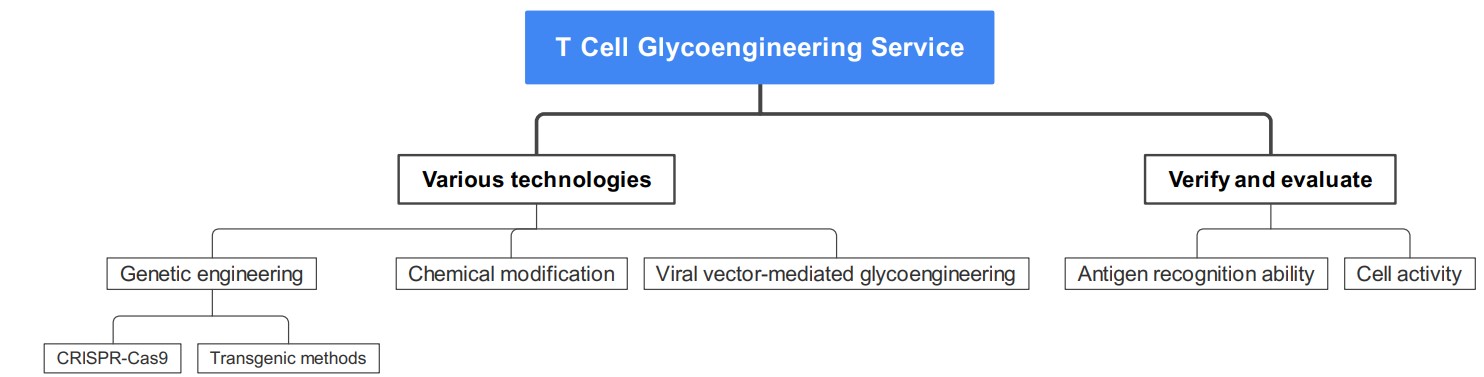
We provide T cell glycoengineering services through a variety of technologies, including but not limited to the following:
We use gene editing technology (such as CRISPR-Cas9) or transgenic methods to introduce specific sugar synthesis-related genes into T cells so that they can express target sugar groups. CRISPR-Cas9 gene editing technology can carry out targeted and precise genetic modification of T cells to regulate the glycosyl structure on the cell surface, providing important tools and methods for personalized treatment and research. According to the sequence of the target gene, we design sgRNA to guide the Cas9 protein to cleave the target gene at a specific site, and then through plasmid construction, transfection, gene editing, and other steps to achieve gene knockout, insertion or modification, thereby changing the glycosyl structure of T cells.
We offer T cell glycoengineering services through chemical modification, a flexible and efficient approach that provides custom cell engineering solutions for research and therapy. We add pre-synthesized chemical modifiers to the T cell culture medium to enable them to bind to the cell surface to form new glycosyl structures. The treated T cells are then washed and purified, and appropriate techniques are used to verify whether the glycosyl modification is successful, such as mass spectrometry, flow cytometry, etc.
-
Viral vector-mediated glycoengineering
We use modified viral vectors to deliver genes related to sugar synthesis to T cells, guiding them to synthesize specific sugar groups. First, we insert the target gene into an appropriate viral vector, such as adenovirus or retrovirus, and then use the constructed viral vector to infect T cells so that the target gene can be expressed and achieve glycosylation modification.
In addition, we also use various technologies to detect and verify whether T cells successfully synthesize the target glycosyl structure, and evaluate the functional changes of glycoengineered T cells, such as antigen recognition ability, cell activity, etc., to confirm the effect of glycosylation modification.
Creative Biolabs focuses on the fields of biotechnology and cell engineering, which has the advanced technology and facilities to provide T cell glycoengineering services. Please contact us if you are interested in our cell line glycoengineering services.
Published data
Chimeric antigen receptor (CAR) T-cell therapy is an emerging immunotherapy that can specifically target tumor cells. However, the widespread use of this therapy is limited by production costs. Therefore, to improve the applicability of CAR-T cell therapy, appropriate strategies need to be developed to enhance its efficacy and reduce production costs. Studies have shown that E-selectin can promote bone metastasis of cancer cells, and the ability of CAR-T cells to home to sites rich in E-selectin is of great significance for accurately targeting a variety of malignant tumors. In this study, the authors conducted detailed biochemical and adhesion experiments to explore the binding force between CAR-T cells and E-selectin. The results showed that by glycoengineering the cell surface, CAR-T cells were effectively and specifically guided into endothelial tissue expressing E-selectin. This glycoengineering technology significantly reduced the burden required for cell expansion, which was expected to achieve lower cost and higher efficacy CAR-T cell therapy.
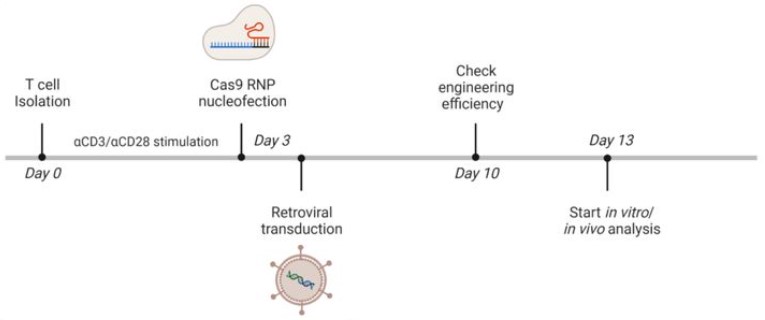 Fig.1 Glycoengineering scheme and results of CAR T cells.1
Fig.1 Glycoengineering scheme and results of CAR T cells.1
FAQ
Q1: How is your T cell glycoengineering technology different from traditional T cell therapies?
A1: Glycoengineering technology can directionally regulate the biological functions of T cells. Compared with traditional T cell therapy, it has a more precise and effective regulatory effect, making the treatment more personalized and efficient.
Q2: How to choose the appropriate glycosyl modification scheme?
A2: We provide personalized modification plans according to the client's research goals and T cell types, and make selections taking into account factors such as research needs, expected effects, and safety.
Q3: How effective are modified T cells in animal models?
A3: We usually conduct animal model experiments to evaluate the efficacy of modified T cells, including anti-tumor effects, immune responses, etc., and provide clients with detailed experimental results and analysis.
Customer Review
Custom solutions
"Creative Biolabs's technical experts custom a glycosyl modification plan for my project research, taking into account my specific experimental needs and goals, demonstrating their personalized service capabilities and professionalism."
Data analysis
"Creative Biolabs provided detailed data analysis and result reports, combined with clear charts and explanations, which helped me fully understand the impact of modifications on T cell functions, and provided important ideas for my future project research."
Reference
-
Mondal, Nandini, et al. "Glycoengineering of chimeric antigen receptor (CAR) T-cells to enforce E-selectin binding." Journal of Biological Chemistry 294.48 (2019): 18465-18474. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services


 Fig.1 Glycoengineering scheme and results of CAR T cells.1
Fig.1 Glycoengineering scheme and results of CAR T cells.1

