Glycomic Profiling Service
Glycoproteins participate in various biological processes, but glycoproteins analysis is a big challenge because of the low abundance and heterogeneity of glycans. As a forward-looking company as well as a leading customer service provider, Creative Biolabs has successfully explored a series of innovative glycomic profiling platforms to help our clients identify an entire set of N- and O-glycans with high sensitivity and specificity in the biological material. We are confident to help you get milestone success in your glycoprotein project.
Background of Glycomic Profiling
Glycosylation a fundamental post-translational modification, which plays an important role in many physiological and pathological processes. Glycoprotein structure analysis allows characterization of important biological molecules and would be of great diagnostic and clinical importance. As an important part of glycoprotein structure analysis, glycomic profiling has attracted a lot of attention.
Glycomic profiling can identify an entire set of N-, and O-glycans present in a diverse range of biological material, including secretions, cell lines, tissues, and organs, which refers to the collection, analysis, and exploitation of glyco-biomaterial at the glycome level. Profiling of glycomic changes in a cell or organism level can be used to provide a general overview on the glycome, the total glycosylation pattern of glycoproteins, glycolipids, or other types of biomolecules, which is increasingly important in searching biomarkers for diseases. N-glycomic and O-glycomic profiling are two common types of analysis. These glycomic strategies are based on rapid, high-sensitivity mass spectrometric (MS) strategies, combined with electrospray (ES) or MALDI tandem mass spectrometry (MS/MS) sequencing and gas chromatography (GC)-MS linkage analysis.
N-Glycomic Profiling in Creative Biolabs
N-glycosylation usually occurs on the asparagine residue of proteins that shuttle via the secretory pathway. As a good complement to genomic and proteomic studies, N-glycomic profiling is widely used in the analysis of the entire repertoire of N-glycans of an organism such as glycans attached to proteins and lipids, glycosaminoglycans and polysaccharides, including genetic, physiologic, pathologic, and other aspects. Moreover, N-glycomic profiling has the diagnostic potential for different stages of cancers and various other disease states, which could provide new biomarkers. Technologies for N-Glycomic profiling in Creative Biolabs including but not limited to:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a high-throughput method to analyze the structural characterization of glycosylated compounds with high sensitivity and robustness. MALDI-TOF MS can identify the entire set of N-glycans expressed by plasma/serum, cell, tissue or organism. During the detection, all glycans attached to the proteins will release by enzymatic digestion, and then separated by hydrophilic chromatography and finally quantitatively profiled with MALDI-TOF MS system.
-
Liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) is a useful tool to analyze N-Glycomic. This protocol permits the detection of permethylated N-glycans in large tissue samples.
O-Glycomic Profiling in Creative Biolabs
O-glycosylations includes two common types, namely O-acetylgalactosamine (O-GalNAc) and O-acetylglucosamine (O-GlcNAc), among which, O-GalNAc is usually attached to the hydroxyl group of the protein serine or threonine residues through an α-linkage, while O-GlcNAc is attached through a β-linkage. Unlike N-glycans, there is no known O-linked amino acid consensus sequence yet. O-Glycomic profiling is an important part of genomic and proteomic studies.
Technologies for O-Glycomic profiling in Creative Biolabs including but not limited to:
-
MALDI-TOF MS is also the most common method for O-Glycomic profiling. MALDI-TOF MS can define the complete repertoire of O-glycans that a cell or tissue produces under specified conditions of time, location, and environment.
Workflow of Glycomic Profiling
 Fig.1 Glycomics workflow by MALDI-TOF MS.1
Fig.1 Glycomics workflow by MALDI-TOF MS.1
Features of our Service
-
Comprehensiveness: collection, analysis, and exploitation of an entire set of N-, and O-glycans at the glycome level.
-
A diverse range of biological material, including secretions, cell lines, tissues, and organs, etc.
-
High sensitivity and specificity: very small amounts of glycoproteins can be analyzed accurately.
-
Stability and consistency: minimized batch-to-batch variations.
-
Competitive prices with quality service.
-
Best after-sale service.
Working with Us to Promote Your Success!
Creative Biolabs has an experienced team of dedicated scientists offering a wide range of glycomic analysis. Our team has successfully completed a lot of projects for glycomic and proteomic analysis. We will provide reliable results to drive your scientific research. We can also customize our offering to meet your specific project needs. If you are interested, please contact us for more information.
Published data
Glycosylation changes of serum proteins play a vital role in pregnancy. Pregnancy causes increased sialylation and galactosylation of N-glycans linked to the Fc domain of immunoglobulin G (IgG), thereby regulating pro-inflammatory and anti-inflammatory responses and ensuring tolerance between mother and fetus. However, the changes in serum N-glycome and sialic acid linkage during pregnancy are still unclear. Therefore, in this study, the researchers developed a high-throughput MALDI-TOF-MS detection method, to conduct a detailed analysis of the total serum N-glycome (TSNG) of 29 healthy women before and after pregnancy, and distinguished α2,6- and α2,3-linked sialic acid derivatives. The results showed that during pregnancy, the sialylation and galactosylation of TSNG changed. The levels of α2,3 and α2,6-linked sialic acid increased, while they decreased after delivery. This MALDI-TOF-MS assay could identify each type of N-glycan in serum with higher confidence and distinguish isoforms. This rapid and reliable assay could be used for high-throughput analysis of glycosylation changes in total serum samples during pregnancy.
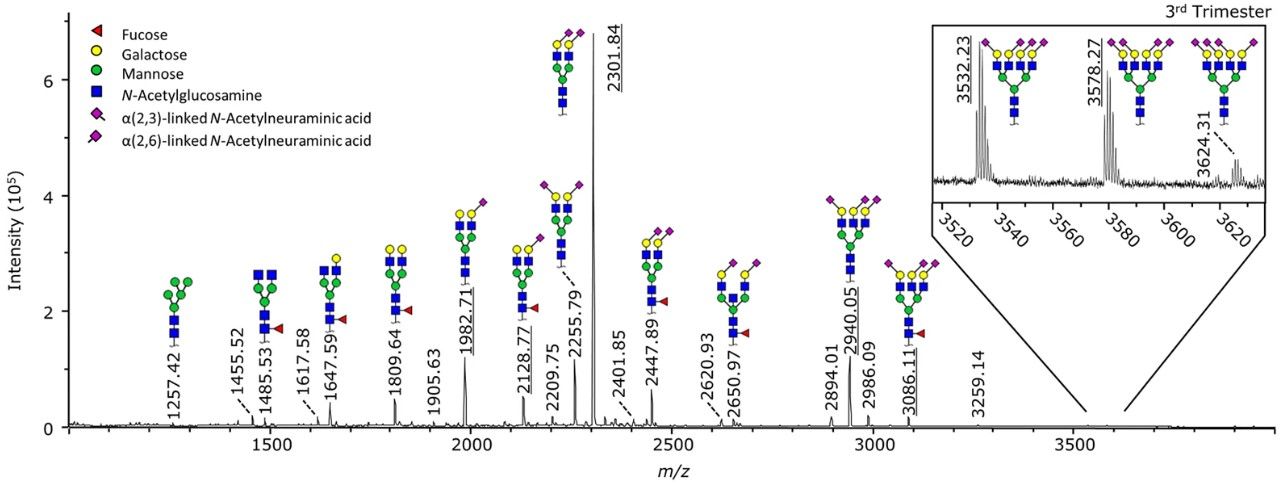 Fig.2 Serum N-glycome profile.1
Fig.2 Serum N-glycome profile.1
FAQs
Q1: How do you ensure high sensitivity and specificity in the glycomic analysis process?
A1: In the process of glycomics analysis, we first release all bound sugar chains by enzymatic cleavage, then separate and purify them by hydrophilic chromatography, and finally use the MALDI-TOF MS system for quantitative analysis. This process ensures high sensitivity and high specificity, because the enzymatic cleavage process can completely release sugar chains, and the hydrophilic chromatography can effectively separate different sugar chains, thereby ensuring the accuracy of MS analysis.
Q2: What experience and technical advantages do you have in dealing with extremely low-content glycoprotein samples?
A2: We have extensive experience in dealing with low-content glycoprotein samples. By using highly sensitive mass spectrometers and optimized sample preparation methods, we accurately analyze and identify trace glycoprotein samples. Our technology can reduce sample loss, and through optimized hydrophilic chromatography and efficient enzymatic cleavage methods, we ensure the stability and accuracy of low-volume samples during analysis.
Q3: Is your analytical technology applicable to different types of biological samples?
A3: Yes, our analytical techniques apply to a variety of biological samples, including cell lines, secretions, organs, and tissues. We use specific sample preparation methods to ensure the accuracy and sensitivity of the analysis when processing different sample types. Whether it is a complex tissue sample or a simple cell sample, we can provide reliable N- and O-glycomics analysis.
Customer Review
Accurate And Efficient Glycomics Service
"With the support of Creative Biolabs, our glycomics research project has made a breakthrough. The N-glycan and O-glycan analysis services they provide through MALDI-TOF MS and LC-ESI-MS technology are not only accurate but also very fast, which greatly improves our research efficiency."
Comprehensive Glycomics Analysis
"Creative Biolabs conducts comprehensive characterization and quantitative analysis of N-glycans through sensitive MS technology. The analysis results they provide are very detailed, revealing many glycosylation modifications that we have not noticed before, which greatly enriches our understanding of the samples."
Reference
-
Jansen, Bas C., et al. "Pregnancy-associated serum N-glycome changes studied by high-throughput MALDI-TOF-MS." Scientific reports 6.1 (2016): 23296. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Glycomics workflow by MALDI-TOF MS.1
Fig.1 Glycomics workflow by MALDI-TOF MS.1
 Fig.2 Serum N-glycome profile.1
Fig.2 Serum N-glycome profile.1



