Glycosylation Site Mapping
Creative Biolabs is a forward-looking company as well as a leading custom service provider in the field of glycoprotein analysis. Based on our extensive experience and advanced platform, we have won a good reputation among our worldwide customers for successfully accomplishing numerous challenging projects in glycosylation site mapping. We are glad to help you get milestone success in your glycoprotein structure analysis project.
Importance of Glycosylation Site Mapping
Glycosylation site mapping helps the identification of a glycosylation site, which can provide an indication of the function of that glycan. In addition, site-specific glycosylation analysis and assignment of a certain structure to a specific site can illuminate the protein’s glycosylation profile, microheterogeneity, and its activity. Glycosylation has great importance in the development of biological drugs because their glycan chains markedly affect product stability, activity, antigenicity, and pharmacodynamics. Glycosylation site mapping mainly contributes to glycan microheterogeneity.
N-Linked Glycosylation Mapping in Creative Biolabs
N-glycosylation usually occurs on the asparagine residue of proteins that shuttle via the secretory pathway. N-linked glycosylation mapping characterizes the site-specific N-glycosylation including N-glycosylation site occupancy and site-specific glycan structure, which is important for the understanding of glycoprotein biosynthesis and function. Through glycosylation mapping, N-glycosylation site quantification can also be identified, helping reveal the critical role of macroheterogeneity in a variety of biological properties. There are a series of techniques we can provide to perform N-linked glycosylation mapping.
Technologies for N-linked glycosylation mapping in Creative Biolabs including but not limited to:
-
RP-HPLC
Reversed-phase high-performance liquid chromatography (RP-HPLC) can analyze the resulting peptide and glycopeptides fragments after digestion of a glycoprotein with specific proteases after reduction and alkylation. RP-HPLC combines on-line mass spectrometry to form a useful tool - RP-HPLC-MS. This method is semi-quantitative because different glycopeptides will ionize differently in a mass spectrometer.
-
LC-ESI-MS
Liquid chromatography-electrospray ionisation mass spectrometry (LC-ESI-MS) is a hyphenated mass spectrometry technique that combines the resolving power of HPLC separation with high mass accuracy of a mass spectrometer. It provides unparalleled sensitivity and selectivity for detection and quantification of site-specific glycan structure.
-
HILIC-SPE-MS
Solid-phase extraction using hydrophilic interaction liquid chromatography mass spectrometry (HILIC-SPE-MS) has become increasingly popular in the glycosylation site mapping because HILIC-SPE-MS is more selective for glycosylated species and is not biased toward particular glycan types. Furthermore, using that technique requires no chemical alteration of glycans.
-
ESI-CID-MS/MS
Electrospray-ionization, collision-induced dissociation, tandem mass spectrometry (ESI-CID-MS/MS) allows for fragmentation of glycan species to be observed predominantly at the glycosidic linkages while the peptide backbone remains intact.
O-Linked Glycosylation Mapping in Creative Biolabs
O-GalNAc is attached to the hydroxyl group of the protein serine or threonine residues through an α-linkage, while O-GlcNAc is attached through a β-linkage. Determining occupied O-linked glycosylation sites is generally much more difficult than site-specific identification of N-linked glycosylation. There is no known amino-acid consensus sequence for O-linked glycans, and there is normally significant heterogeneity with O-glycosylated protein regions, both in the number of glycans and the extent of their occupancy. There are also a number of approaches that are based on the signal intensity of glycopeptide/peptide ions for O-linked glycosylation mapping, which is consistent with N-linked glycosylation mapping.
Technologies for O linked glycosylation mapping in Creative Biolabs including but not limited to:
Features of our Service
-
High sensitivity and specificity
-
High batch-to-batch consistency
-
Competitive prices with quality service
-
Best after-sale service
Working with Us to Promote Your Success!
Creative Biolabs is a leader in the field of glycoprotein analysis and has successfully completed a lot of therapeutic glycoprotein projects. We have experienced experts and advanced platforms that can provide excellent services for glycosylation site mapping. We offer a whole set of glycosylation site mapping service to help you get landmark development. We can also customize our offering to meet your specific project needs. If you are interested, please contact us without hesitation.
Published data
Glycosylation of proteins is a key form of post-translational modification. Glycosylation can give modified proteins new functions. Therefore, analyzing the glycosylation sites of glycoproteins is an important means to evaluate the biological functions of target glycoproteins. At present, scientists have analyzed the N-glycosylation of various plants by combining lectin capture, HILIC, and highly sensitive MS technology. The germination of rice seeds is regulated by various proteins, including N-glycoproteins. In this study, the authors used rice named Oryza sativa L. as the research object and analyzed the N-linked glycosyl sites in rice on a large scale. The authors first collected and separated the germinated embryos from rice seeds, and placed the embryo samples in lysis buffer and trypsin digestion solution for protein extraction and digestion. Subsequently, N-glycopeptides were enriched and deglycosylated by HILIC. LC-MS analysis was then performed, and the MS data were parsed using software and databases to analyze the N-glycosylation sites of rice embryos. The results showed that 242 glycosylation sites were identified in the proteins of embryo samples. N-glycosylation was mainly involved in carbohydrate metabolism, cell wall remodeling and modification, Brassinosteroids (BRs) signal transduction, and other processes during embryo germination, which may be the main mechanism for regulating embryo germination.
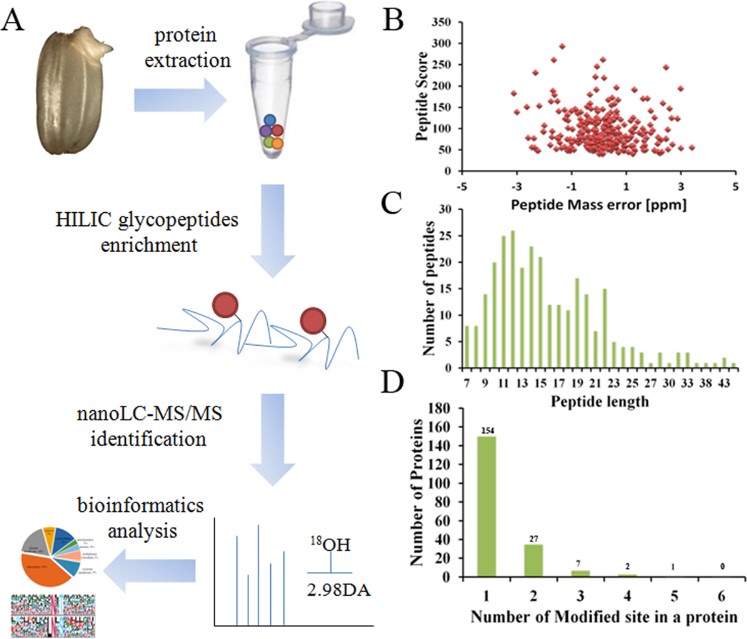 Fig.1 Workflow of the glycosylation sites mapping.1
Fig.1 Workflow of the glycosylation sites mapping.1
FAQs
Q1: What technology do you use to identify glycosylation sites?
A1: We usually use MS to identify glycosylation sites. We digest the protein in the sample into peptides and analyze these peptides by MS to determine the specific location where the glycosylation modification occurs.
Q2: Does your glycosylation site mapping cover all types of glycosylation modifications?
A2: Our service usually detects and identifies multiple types of glycosylation modifications, including N-glycosylation, O-glycosylation, etc. However, it is necessary to select the appropriate experimental protocol according to specific needs and sample characteristics.
Q3: Can your glycosylation site mapping provide quantitative information?
A3: Yes, we provide quantitative information on glycosylation sites by combining MS technology and appropriate standard curve methods, which helps clients understand the degree of glycosylation modification at different sites.
Customer Review
Identify And Quantify Target Glycosylation Sites
"We commissioned the Creative Biolabs team to perform glycosylation site mapping services and obtained satisfactory results. They used advanced mass spectrometry technology to successfully identify and quantify the glycosylation sites of interest to us, solving key problems in our research."
Efficient Glycosylation Analysis Supports Client Projects
"The glycosylation site mapping service provided by Creative Biolabs is very professional and efficient. Through their technical means, we successfully determined the glycosylation modification locations of multiple proteins in our samples and obtained detailed reports, which is of great significance to our further research work."
Reference
-
Ying, Jiezheng, et al. "Mapping the N-linked glycosites of rice (Oryza sativa L.) germinating embryos." PLoS One 12.3 (2017): e0173853. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services:
- Glycan Profiling
- Glycomic Profiling
- Glycan Sequencing

 Fig.1 Workflow of the glycosylation sites mapping.1
Fig.1 Workflow of the glycosylation sites mapping.1


