Comprehensive N-glycan Profiling of Cetuximab Biosimilar Candidates
Cetuximab is a recombinant monoclonal antibody that has been applied for various cancer treatments for many years. Biosimilar candidates for cancer therapies development is of great significance for humans. Based on first-in-class technology platforms, Creative Biolabs has made great achievements in glycoprotein investigations. We are proud to provide high-quality services in N-glycan profiling of biosimilar candidates at the most competitive cost.
Background of Cetuximab Biosimilar Candidates
Therapeutic recombinant monoclonal antibody (mAbs) drugs have emerged as a clinically important drug class, which were mainly used for cancer treatment. There are more than 30 therapeutic antibodies have been approved for clinical use. Cetuximab, a therapeutic mAb drug approved by FDA in 2009, actually is an epidermal growth factor receptor inhibitor that has been applied for clinical treatment of metastatic colorectal cancer, metastatic non-small cell lung cancer, and head and neck cancer. Recent years, studies on biosimilar candidate are increasing. The development of biosimilar candidates of cetuximab is becoming a trend mainly due to:
-
Treatment of cancers with good therapeutic effect and little or no effect
-
Lucrative targets
-
Patent protection has expired
-
The expensiveness of the production and characterization
Why N-glycan Profiling?
-
The attachment N-glycans on mAbs or glycosylation of mAbs is considered to play significant roles in many biological and therapeutic processes such as cell-cell adhesion, cell surface receptor recognition, and antibody-dependent cellular cytotoxicity.
-
A part of antibodies can’t function without glycans due to that glycans are essential for antibody binding to all Fc gamma receptors.
-
Previous antibody glycan analysis is mainly performed by the 2-aminobenzamide hydrophilic interaction liquid chromatography (HILIC) method after glycans released from antibodies, which has limited use for screening a large number of samples
Therefore, N-glycan profiling analysis of the mAbs or biosimilar candidate is becoming an increasingly critical process in antibody characterization and regulatory evaluation. Creative Biolabs has accumulated extensive experience in N-glycan profiling based on powerful technologies.
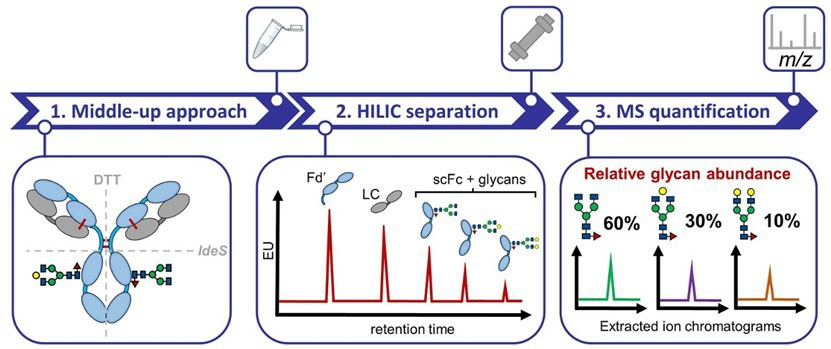 Fig.1 Quantitative N-glycan analysis of mAbs.1
Fig.1 Quantitative N-glycan analysis of mAbs.1
Creative Biolabs has successfully developed several effective strategies in comprehensive N-glycan profiling of biosimilar candidates. If you need a professional scientific research staff and a full range of services on N-glycan profiling, just feel free to contact us for more detailed information.
Reference
-
Duivelshof, Bastiaan L., et al. "Quantitative N-glycan profiling of therapeutic monoclonal antibodies performed by middle-up level HILIC-HRMS analysis." Pharmaceutics 13.11 (2021): 1744. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services:

 Fig.1 Quantitative N-glycan analysis of mAbs.1
Fig.1 Quantitative N-glycan analysis of mAbs.1


