Creative Biolabs is a well-recognized expert in the field of antibody generation and production. Especially, we have launched a series of in vitro diagnostic (IVD) antibody development services for different infections and diseases, including sepsis. Scientific progress has resulted in the discovery of novel disease biomarkers to fulfill the need for quicker, more specific and more accurate diagnosis of sepsis. Particularly, we provide IVD antibody development services against the monocyte chemotactic protein 2 (MCP-2) marker.
Introduction of MCP-2
MCP-2, also known as Chemokine ligand 8 (CCL8), is a small cytokine that belongs to the CC chemokine family. The MCP-2 protein is produced as a precursor containing 109 amino acids (aa), and then cleaved to be a mature protein containing 75 aa. It is chemotactic for and activates many different immune cells, including cells that are implicated in allergic responses (mast cells, eosinophils, and basophils) and cells that are involved in the inflammatory responses (monocytes, T cells, and NK cells). MCP-2 functions by binding to several different cell surface receptors as ligands, including CCR1, CCR2B, CCR3, and CCR5.
 Fig.1 Structures of MCP-2.1
Fig.1 Structures of MCP-2.1
MCP-2 Biomarker for Sepsis Prognosis & Diagnosis
Biomarkers have an important place in the early diagnosis and stratification of the severity of sepsis because they can indicate the presence or absence or severity of sepsis, differentiate bacterial from viral and fungal infection, and systemic sepsis from local infection, increasing the possibility of starting timely and specific treatment. Cytokines are a group of compounds that have been widely assessed as potential biomarkers of sepsis prognosis and diagnosis. One of them is the MCP-2, which has been discovered to be upregulated in patients with sepsis. Together with MCP-1, MCP-2 has been evaluated as a prognostic factor of sepsis. They have been found to be distinguished between survivors and non-survivors at 28 days. However, more clinical and experimental studies are needed to help better evaluate the utility, specificity, and sensitivity of MCP-2 as a biomarker for sepsis.
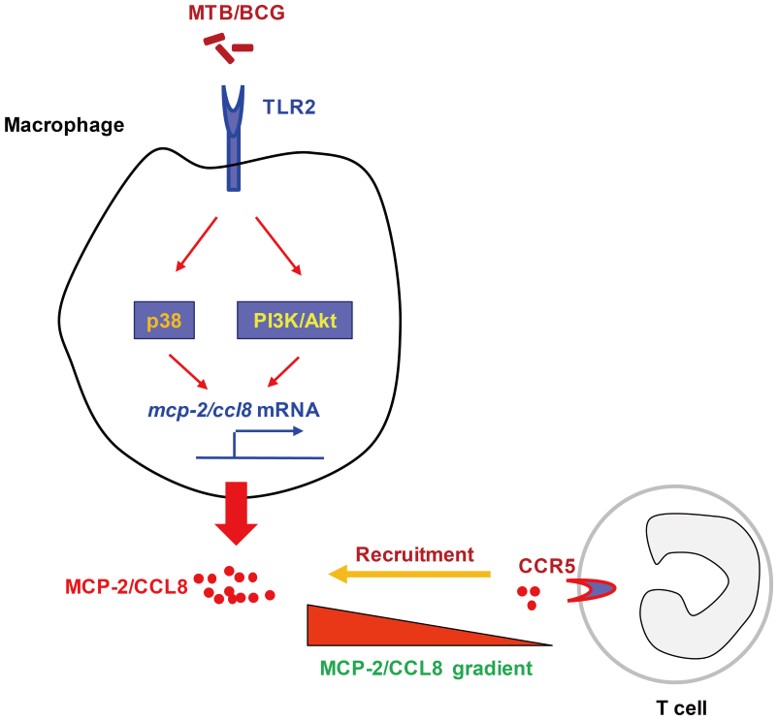 Fig.2 MCP-2/CCL8 expression in macrophages induced by mycobacteria.2
Fig.2 MCP-2/CCL8 expression in macrophages induced by mycobacteria.2
IVD Antibody Development Services for MCP-2 Biomarker
Creative Biolabs has built a versatile IVD platform for not only the development of high-quality antibodies for diagnostic use but also antibody & antigen conjugation and IVD kit development. We provide polyclonal, monoclonal, and recombinant antibody development services against the MCP-2 biomarker. Besides, we offer expertise in diagnostic immunoassay development of different formats, such as ELISA, lateral flow assays, western blot, immunohistochemistry, etc. Customized services are provided to meet every specific requirement.
Creative Biolabs has focused on the development of IVD antibodies against a large number of biomarkers of sepsis. Supported by our highly specialized scientists and advanced platform, we are confident in providing services with the best quality and the most competitive prices. If you are interested in our services, contact us for more information.
References
- From Wikimedia: By Jmol Development Team, GPL, without modification, https://commons.wikimedia.org/wiki/File:1ESR.pdb1.png.
- Liu, Haipeng, et al. "Induction of CCL8/MCP-2 by mycobacteria through the activation of TLR2/PI3K/Akt signaling pathway." PLoS One 8.2 (2013): e56815. under Open Access license CC BY 4.0, without modification.
For Research Use Only.

