As a leading CRO of antibody generation and development, Creative Biolabs offers application-specific antibody development services to global clients. With experienced scientists and advanced technology, we have established a series of high-quality in vitro diagnostic (IVD) antibody development services against biomarkers of different diseases. Here, we introduce our IVD antibody development services for procalcitonin marker.
Procalcitonin
PCT is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It arises once preprocalcitonin is cleaved by endopeptidase. PCT is the pro-peptide of calcitonin, a 116-peptide molecule with a molecular weight of 13 kDa. It can be synthesized by C cells of the thyroid gland and released from leukocytes of the peripheral blood. In general, the level of PCT in the blood stream of healthy individuals is below the limit of detection (0.01 µg/L) of clinical assays. While individuals are exposed to proinflammatory stimulus, especially of bacterial origin, the level of PCT is increased. So PCT often defined as an acute phase reactant. Recent studies have been shown that PCT can act as a sepsis biomarker to help with diagnosing/ ruling out sepsis and to guide the initiation and cessation of antibiotics. It has also demonstrated that PCT has a strong correlation with medullary thyroid carcinoma (MTC), representing a promising MTC tumor marker.
 Fig. 1 Structure of procalcitonin.1
Fig. 1 Structure of procalcitonin.1
The Role of Procalcitonin in Sepsis
An ideal biomarker should possess high diagnostic accuracy, for an early and rapid diagnosis. PCT is a recently discovered biomarker that fulfills many of these requirements especially in comparison to conventional and widely used other biomarkers that have demonstrated superior diagnostic accuracy for a variety of infections, including sepsis. PCT is helpful for early detection of sepsis as well as to monitor the antimicrobial treatment regimen. In fact, PCT can be a useful tool for antimicrobial stewardship and its utilization may safely lead to significant reduction of the unnecessary administration of antimicrobial therapy. Laboratories and clinicians must comprehend the precincts of the present microbiological methods and the need for highly sensitive biomarker assays to facilitate accurate diagnosis and goal-directed therapy in patients suspected of sepsis.
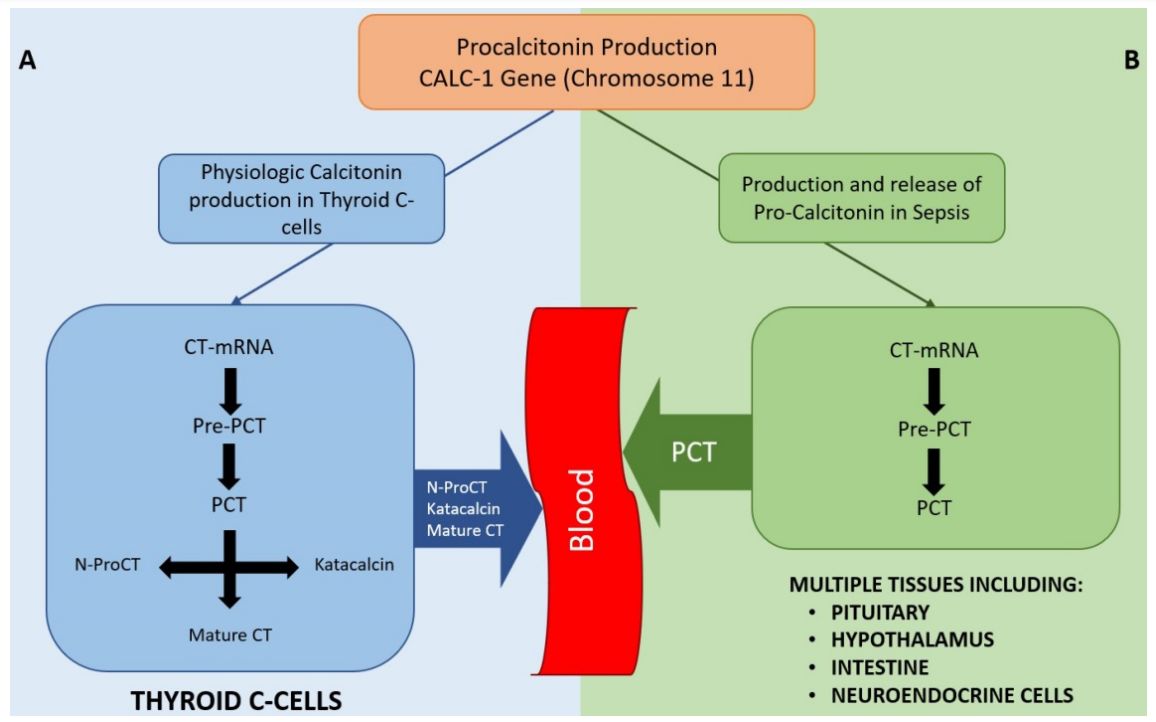 Fig. 2 Physiological production of Calcitonin and production of PCT in sepsis states.2,3
Fig. 2 Physiological production of Calcitonin and production of PCT in sepsis states.2,3
The Role of Procalcitonin in Medullary Thyroid Cancer
Medullary thyroid carcinoma (MTC) is a rare but aggressive thyroid malignancy, which originates from the thyroid parafollicular cells (C cells) and accounts for about 3% of thyroid cancers. The major clinical symptom of MTC is diarrhea, flushing, a thyroid nodule and enlarged cervical lymph nodes. It is accepted that the gold-standard biomarker for MTC diagnosis and follow-up is calcitonin (CT), with great sensitivity and specificity. But it has several limitations because of the biphasic half-life, sensitivity to relatively rapid degradation by serum proteases and various different immunoreactive isoforms. Now, procalcitonin (PCT), the prohormone of calcitonin, has been recognized as a stable and burgeoning biomarker in the diagnosis and follow-up of MTC, because of the similar distribution of values as calcitonin and strong correlation with MTC.
IVD Antibody Development Services Targeting Procalcitonin Marker
In recent years, IVD technologies are undergoing rapid development. IVD antibodies have been widely used in the diagnosis of numerous diseases including but not limited to sepsis. Antibody-based immunoassays are the most generally used diagnostic assays for the detection of biomolecules. With advanced technology and professional scientists, Creative Biolabs is capable of offering a full range of IVD antibody development services against various markers for global customers. Besides, Creative Biolabs provides one-stop diagnostic immunoassay development services, covering feasibility analysis, assay design, assay protocol establishment, validation, and production.
For more detailed information, please feel free to contact us or directly send us an inquiry.
References
- From Wikipedia: By Abargmann987, Own Work, CC BY-SA 4.0, https://commons.wikimedia.org/wiki/File:A_3D_cartoon_of_calcitonin.png
- Paudel, Robin, et al. "Procalcitonin: A promising tool or just another overhyped test?." International Journal of Medical Sciences 17.3 (2020): 332.
- under Open Access license CC BY 4.0, without modification.
For Research Use Only.

